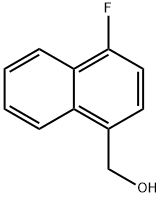
(4-Fluoronaphthalen-1-yl)methanol synthesis
- Product Name:(4-Fluoronaphthalen-1-yl)methanol
- CAS Number:79996-88-6
- Molecular formula:C11H9FO
- Molecular Weight:176.19
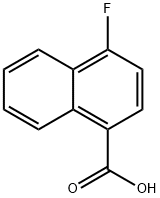
573-03-5
112 suppliers
$22.00/1g

79996-88-6
27 suppliers
inquiry
Yield:79996-88-6 72%
Reaction Conditions:
Stage #1: 4-fluoro-1-naphthoic acidwith N-ethyl-N,N-diisopropylamine;isobutyl chloroformate in tetrahydrofuran at -10; for 0.5 h;
Stage #2: with sodium tetrahydridoborate in tetrahydrofuran;water monomer at 20; for 0.333333 h;
Stage #3: with hydrogenchloride in water monomer;
Steps:
29.a
a) 4-fluoro-1-naphthoic acid (1.5 g. 7.88 mmol) and N, N-diisopropylethylamine (1.54 mL, 8.67 mmol) were dissolved in 50 mL of anhydrous THF and the solution cooled to -10 °C. To the cooled solution, isobutylchloroformate (1.12 mL, 8.67 mmol) was added drop wise and stirred at for 30 minutes. The solution was filtered into a flask containing sodiumborohydride (1.19 g, 31.54 mmol) dissolved in a minimal amount of water. The foaming solution was stirred for an additional 20 minutes at RT. The solution was slowly made acidic with 1 N HCl and partitioned between EtOAc and water. The organic layer was separated and washed sequentially with 1) sat. NaHCO3, 2) sat. brine then dried over MgSO4 filtered, and concentrated. The residue was purified by column chromatography (1:1 EtOAc: Hexane) and the volatiles removed under reduced pressure to give the title compound as a pinkish white solid (1.0 g, 72% yield). MS m/z 159 (M+H-H20)+.
References:
WO2004/22539,2004,A1 Location in patent:Page 31
172033-73-7
98 suppliers
$59.00/500mg

79996-88-6
27 suppliers
inquiry

341-41-3
164 suppliers
$9.00/1g

79996-88-6
27 suppliers
inquiry
316-68-7
90 suppliers
$19.00/1g

79996-88-6
27 suppliers
inquiry