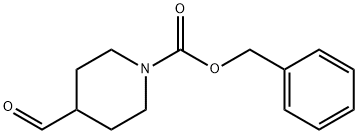
4-FORMYL-N-CBZ-PIPERIDINE synthesis
- Product Name:4-FORMYL-N-CBZ-PIPERIDINE
- CAS Number:138163-08-3
- Molecular formula:C14H17NO3
- Molecular Weight:247.29
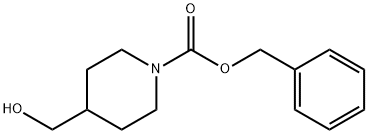
122860-33-7

138163-08-3
The general procedure for the synthesis of 4-formyl-N-CBZ piperidine from N-CBZ-4-piperidine methanol (1-CBZ-4-hydroxymethylpiperidine) was as follows: Step 3: Synthesis of benzyl 4-formyl-piperidine-1-carboxylate (3); Oxalyl chloride (59.1 mL, 674 mmol) was dissolved in dichloromethane (500 mL) and the solution was cooled to -78 °C. The solution was then dissolved in dichloromethane (500 mL). Dimethyl sulfoxide (68.3 mL, 963 mmol) was added slowly with stirring and stirring was continued for 15 minutes. Subsequently, benzyl 4-hydroxymethyl-piperidine-1-carboxylate (2) (120 g, 481 mmol) dissolved in dichloromethane (500 mL) was added. The reaction mixture was slowly warmed to -55 °C and kept at this temperature for 15 minutes. After that, the mixture was again cooled to -78 °C and a solution of dichloromethane (250 mL) of triethylamine (205 mL, 1443 mmol) was slowly added. The reaction suspension was allowed to warm slowly to room temperature and the reaction was quenched with glacial acetic acid (100 mL). The reaction solution was washed with water and the aqueous phase was back-extracted with dichloromethane (2 x 200 mL). All organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated to afford benzyl 4-formyl-piperidine-1-carboxylate (119 g, 100% yield).

79-37-8
490 suppliers
$17.67/10gm:

122860-33-7
173 suppliers
$5.00/1g

138163-08-3
192 suppliers
$6.00/1g
Yield:138163-08-3 100%
Reaction Conditions:
with triethylamine in dichloromethane;dimethyl sulfoxide
Steps:
26.c 26c.
26c. Phenylmethyl 4-formylpiperidinecarboxylate To a stirred solution of oxalyl chloride (2M solution in dichloromethane, 10.9 mL, 21.9 mmol) was added DMSO (3.1 mL, 3.4 g, 43.8 mmol) in dichloromethane (6 mL) over a period of 15 minutes. The product of Example 26b (4.4 g, 17.5 mmol) in dichloromethane (7 mL) was then added at -78° C. over a period of 15 minutes. The resultant solution was stirred at -78° C. for 1 hour and then triethylamine (12.2 mL, 8.86 g, 87.5 mmol) was added, dropwise, over a period of 15minutes. The mixture was further stirred at -78° C. for 30 min and then at 0° C. for 15 min. The reaction mixture was quenched with water and extracted with dichloromethane. The combined organic phase was washed with 1% HCl, water, dried over sodium sulfate, filtered and evaporated to give the title compound (4.4 g, 100%) which was used in the next step without purification. 1H NMR (300 MHz, CDCl3) δ9.65 (s, 1H), 7.28-7.38 (m, 5H), 5.12 (s, 2H), 4.04 (br d, J=13.1 Hz, 2H), 2.97-3.06 (m, 2H), 2.38-2.45 (m, 1H), 1.88-1.92 (m,2H), 1.52-1.64 (m, 2H). 13C NMR (75 MHz, CDCl3) δ202.7, 155.2, 136.7, 128.5, 128.6, 127.9, 67.2, 47.8, 43.0, 25.1. LRMS (APIMS) m/z 248 (MH+).
References:
Fang, Xinqin;Garvey, David S.;Gaston, Ricky D.;Lin, Chia-En;Ranatunga, Ramani R.;Richardson, Stewart K.;Wang, Tiansheng;Wang, Weiheng;Wey, Shiow-Jyi US2003/203915, 2003, A1

122860-33-7
173 suppliers
$5.00/1g

138163-08-3
192 suppliers
$6.00/1g
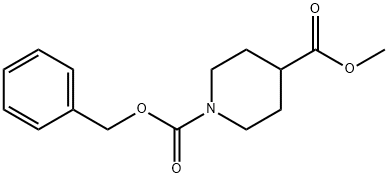
138163-07-2
121 suppliers
$5.00/5g

138163-08-3
192 suppliers
$6.00/1g
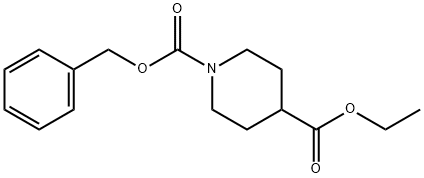
160809-38-1
89 suppliers
$38.00/1g

138163-08-3
192 suppliers
$6.00/1g

148148-48-5
41 suppliers
$92.00/1g

138163-08-3
192 suppliers
$6.00/1g