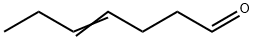
4-HEPTENAL synthesis
- Product Name:4-HEPTENAL
- CAS Number:62238-34-0
- Molecular formula:C7H12O
- Molecular Weight:112.17

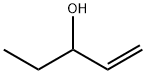
592-42-7
172 suppliers
$22.00/25mL
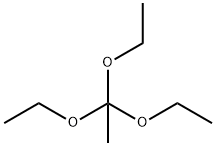
201230-82-2
1 suppliers
inquiry
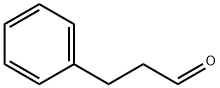
104-53-0
267 suppliers
$6.00/5g
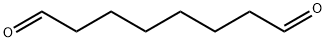
638-54-0
12 suppliers
inquiry
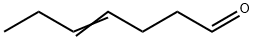
62238-34-0
8 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;triphenylphosphine in water at 80; under 7500.75 Torr; for 3 h;Autoclave;Inert atmosphere;regioselective reaction;
Steps:
2.1. The general procedure of hydroformylation
General procedure: Hydroformylation experiments were carried out in 100 mL (or50 mL) stainless steel autoclaves provided with a manometer, a thermostat, a magnetic stirrer, and a gas inlet/outlet system. The catalyst, Rh(acac)(CO)2 or Rh/PAA, and PPh3 were placed in the autoclave. Then, 1.5 mL of substrate were introduced into the auto-clave under a nitrogen atmosphere. In the case of experiments carried out on water, 1.5 mL of twice distilled water was added. The autoclave was closed, flushed with hydrogen (5 bar) three times,and thereafter pressurized with a synthesis gas (H2: CO = 1:1) to 10 bar (or 14 bar) and heated to 80°C (or 60°C). After the reaction was finished, the autoclave was cooled to room temperature and the residual gases depressurized. The product mixture was immediately analyzed by NMR spectra, the catalyst was removedby StratospheresTMSpe, and the obtained products were identified by means GC and GC-MS.Selected reaction mixtures were analyzed additionally using1Hand13C NMR (Figs. 1-3).
References:
Alsalahi;Trzeciak [Journal of Molecular Catalysis A: Chemical,2016,vol. 423,p. 41 - 48]