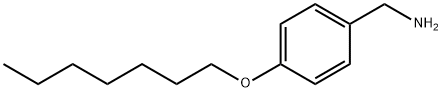
4-HEPTYLOXY-BENZYLAMINE synthesis
- Product Name:4-HEPTYLOXY-BENZYLAMINE
- CAS Number:4950-92-9
- Molecular formula:C14H23NO
- Molecular Weight:221.34

203116-01-2
5 suppliers
inquiry

4950-92-9
5 suppliers
inquiry
Yield:4950-92-9 84%
Reaction Conditions:
with sodium hydroxide;sodium sulfate in tetrahydrofuran;diethyl ether;hexane;water;
Steps:
162.2 step 2
step 2 4-(heptyloxy)benzylamine To a stirred suspension of lithium aluminum hydride (1.928 g, 52.23 mmol) in THF (150 ml) at room temperature under nitrogen was added a suspension of 4-(heptyloxy)benzamide (6.146 g, 26.117 mmol). After refluxing for 22 h, reaction was cooled in an ice bath and quenched in the following order: water (2.09 ml), 15% NaOH (2.09 ml) and water (6.26 ml). The mixture was stirred for 3 h followed by the addition of Na2SO4. The mixture was filtered and concentrated in vac. The residue was taken up in Et2O, filtered, concentrated, and the resulting residue taken up in hexane, filtered, concentrated, and dried to give the desired product as a hazy yellow oil (4.872 g, 84%): 1H NMR (400 MHz, DMSO-d6) δ0.86 (t, J=7 Hz, 3H), 1.22-1.42 (m, 8H), 1.64-1.72 (m, 2H), 1.82 (broad s, 2H), 3.13 (s, 2H), 3.89 (t, J=6.6 Hz, 2H), 6.80-6.84 (m, 2H), 7.17-7.21 (m, 2H); IR (film) 2940, 2850, 1510, 1250 cm-1; mass spectrum [(+)ESI], m/z 222 (M+H)+.
References:
US6214877,2001,B1