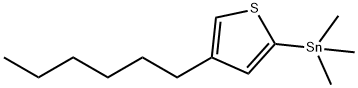
(4-hexylthiophen-2-yl)triMethylstannan synthesis
- Product Name:(4-hexylthiophen-2-yl)triMethylstannan
- CAS Number:154717-22-3
- Molecular formula:C13H24SSn
- Molecular Weight:331.1
1693-86-3
274 suppliers
$10.00/1g

1066-45-1
143 suppliers
$31.50/2g

154717-22-3
18 suppliers
$124.00/1G
Yield:154717-22-3 100%
Reaction Conditions:
Stage #1: 3-hexylthiophenewith n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 1 h;
Stage #2: trimethyltin(IV)chloride in tetrahydrofuran at -78 - 20; for 14 h;
Steps:
I.3.1 (3-1) Synthesis of (4-hexylthiophen-2-yl)trimethylstannane
To 10 mL of dehydrated THF, diisopropylamine (4 mL, 28.5 mmol) was added at 10° C. To the mixture, 2.6 M of n-BuLi (a hexane solution) (9.88 mL, 26.2 mmol) was slowly added by dripping, and the resultant was stirred for 30 minutes at -10° C. Thereafter, a temperature of the resultant was returned to room temperature, to thereby prepare lithium diisopropylamide (LDA). To 200 mL of dehydrated THF, 3-hexylthiophene (4.03 g, 23.8 mmol) was added, and the resultant was stirred for 30 minutes at -78° C. To the resulting mixture, LDA was slowly added by dripping, and the resultant was stirred for 1 hour. To the resultant, trimethyltin chloride (5.69 g, 28.6 mmol) was slowly added, and the mixture was further stirred for 2 hours at -78° C. Thereafter, a temperature of the resultant was returned to room temperature, and the resultant was stirred for 12 hours. To the reaction container, water was added to perform extraction with hexane, the solvent was removed, and vacuum drying was performed, to thereby obtain pale yellow oil (7.92 g, yield: 100%). (0148) 1H NMR (400 MHz, CDCl3): δ7.19 (s, 1H), 7.01 (s, 1H), 2.64 (t, J=7.8 Hz, 2H), 1.66-1.58 (m, 2H), 1.39-1.21 (m, 6H), 0.92-0.81 (m, 3H), 0.36 (s, 9H)
References:
US10062854,2018,B2 Location in patent:Page/Page column 42; 43