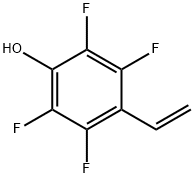
4-Hydroxy-2,3,5,6-tetrafluorostyrene synthesis
- Product Name:4-Hydroxy-2,3,5,6-tetrafluorostyrene
- CAS Number:385422-30-0
- Molecular formula:C8H4F4O
- Molecular Weight:192.11
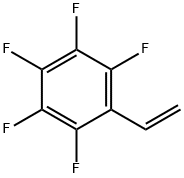
653-34-9
159 suppliers
$8.00/1g

385422-30-0
24 suppliers
$45.00/10mg
Yield: 58%
Reaction Conditions:
Stage #1:2,3,4,5,6-pentafluorostyrene with potassium hydroxide in tert-butyl alcohol for 2 h;Reflux;
Stage #2: with hydrogenchloride in water;tert-butyl alcohol; pH=Ca. 3 at 20;
Steps:
4.3 Synthesis of 2,3,5,6-tetrafluoro-4-vinylphenol (TFSOH)
The synthesis of TFSOH was performed similar to a previously reported procedure [28]. Briefly, FS (20 g, 0.1 mol) was added to a mixture of KOH (30 g, 0.53 mol) in 400 mL of tert-BuOH. The mixture was refluxed for 2 h, cooled down to room temperature and diluted with 1200 mL of water. The alcohol was distilled off and the residue was extracted with diethyl ether (3× 300 mL). The aqueous phase was acidified (pH ∼ 3) with 10 wt.% HCl and was extracted with diethyl ether (3× 300 mL). The organic phase was dried over MgSO4 overnight. The solution was filtered and the diethyl ether was distilled off. The product was purified through a vacuum distillation. Yield: 11.5 g (58%). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 11.62 (OH), 6.59 (CH=CH2), 5.60-5.92 (CH=CH2). FTIR (cm-1): 3400 (OH).
References:
Dimitrov, Ivaylo;Jankova, Katja;Hvilsted, Søren [Journal of Fluorine Chemistry,2013,vol. 149,p. 30 - 35]