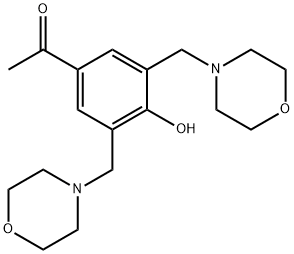
4-Hydroxy-3,5-bis(morpholinomethyl)benzaldehyde synthesis
- Product Name:4-Hydroxy-3,5-bis(morpholinomethyl)benzaldehyde
- CAS Number:1064288-38-5
- Molecular formula:C18H26N2O4
- Molecular Weight:334.41

110-91-8
678 suppliers
$9.00/1g

50-00-0
846 suppliers
$10.00/25g
99-93-4
833 suppliers
$5.00/25g

1064288-38-5
5 suppliers
inquiry
Yield:1064288-38-5 100%
Reaction Conditions:
in 1,4-dioxane at 120; for 0.5 h;Microwave irradiation;Green chemistry;regioselective reaction;
Steps:
3.1. General Synthesis of Mannich Bases (2-5)
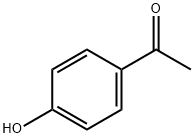
General procedure: To a solution of 4-hydroxyacetophenone (1) (16 mmol, 2.18 g) and formaldehyde (24 mmol or 32 mmnol) in 1,4-dioxane (15 ml), the corresponding secondary amine (morpholine (2), pyrrolidine (3), peridine (4) or diethylamine (5)) was added using the same ratio of formaldehyde. This mixturewas placed in the MW sealed vessel with stirring. The reaction mixture was irradiated for 15-30 minat 120 °C (power 300 W). TLC was used to monitor the progress of the reaction. After consumptionof the starting materials, the vessel was removed and cooled down to room temperature. The reactionmixture was concentrated under reduced pressure and purified using column chromatography.
References:
Aljohani, Ghadah;Said, Musa A.;Lentz, Dieter;Basar, Norazah;Albar, Arwa;Alraqa, Shaya Y.;Al-Sheikh Ali, Adeeb [Molecules,2019,vol. 24,# 3,art. no. 590]

110-91-8
678 suppliers
$9.00/1g

50-00-0
846 suppliers
$10.00/25g

99-93-4
833 suppliers
$5.00/25g

1064288-38-5
5 suppliers
inquiry
![1-[4-HYDROXY-3-(MORPHOLIN-4-YLMETHYL)PHENYL]ETHANONE HYDROCHLORIDE](/CAS/GIF/92041-43-5.gif)
92041-43-5
8 suppliers
inquiry