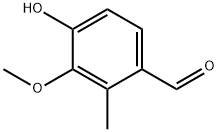
4-Hydroxy-3-Methoxy-2-Methylbenzaldehyde synthesis
- Product Name:4-Hydroxy-3-Methoxy-2-Methylbenzaldehyde
- CAS Number:18102-32-4
- Molecular formula:C9H10O3
- Molecular Weight:166.17
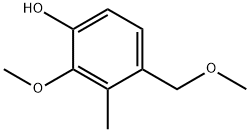
109685-13-4
1 suppliers
inquiry

18102-32-4
10 suppliers
inquiry
Yield:-
Reaction Conditions:
with 2,3-dicyano-5,6-dichloro-p-benzoquinone in dichloromethane;water at 20;
Steps:
1.c
Our synthesis commenced with commercially available vanillyl alcohol 3a. Compound 3a was treated with catalytic amounts of tosyl acid in methanol to afford 3b, presumably via a p-quinone methide intermediate (Scheme 2) . The aromatic methyl group was then installed regiospecifically at the more hindered site through directed lithiation followed by methylation to afford 6. A subsequent 2,3- Dichloro-5, 6-dicyano-l, 4-benzoquinone (DDQ) -mediated oxidation gave rise to 2 -methyl vanillin 7 in 44% overall yield from 3a. Crotylation of the phenolic hydroxyl group, followed by a Claisen rearrangement produced phenol 8. For greater flexibility in later transformations, the phenol was protected either as a benzyl ether 9a or as a MOM ether 9b. Baeyer-Villiger oxidation of these compounds followed by hydrolysis afforded phenols 10a and 10b in 54% and 95% overall yields, respectively, from 8. The resultant phenolic hydroxyl group in each compound was then tosylated to produce 11a and lib in the yields shown. These compounds were hydrof ormylated with acetyl acetonate dicarbonyl rhodium (1) (Rh (CO) 2 (acac) ) and the Billig bis- organophosphite ligand according to Buchwald's protocol, to give exclusively the desired linear aldehydes 12a and 12b in 90% and 82% yields, respectively (Cuny, et al . , J. Am. Chem. Soc. 1993, 115, 2066) . EPO
References:
WO2006/83417,2006,A2 Location in patent:Example 1
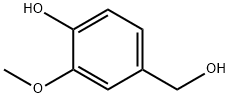
498-00-0
357 suppliers
$21.00/25g

18102-32-4
10 suppliers
inquiry
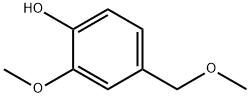
5533-03-9
6 suppliers
inquiry

18102-32-4
10 suppliers
inquiry
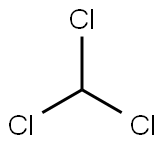
67-66-3
0 suppliers
$33.60/500ml

18102-31-3
22 suppliers
$310.00/100mg

18102-32-4
10 suppliers
inquiry