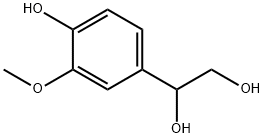
4-HYDROXY-3-METHOXY-D3-PHENYLETHYLENE GLYCOL synthesis
- Product Name:4-HYDROXY-3-METHOXY-D3-PHENYLETHYLENE GLYCOL
- CAS Number:534-82-7
- Molecular formula:C9H12O4
- Molecular Weight:184.19
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

534-82-7
21 suppliers
$65.00/10mg
Yield: 78%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; under 2585.81 Torr; for 2 h;Autoclave;
Steps:
6.1.3.8. 1-(4-Hydroxy-3-methoxyphenyl)ethane-1,2-diol (16)
1-(4-Benzyloxy-3-methoxyphenyl)ethane-1,2-diol 34 (0.106 mmol,0.029 g) was dissolved in MeOH (2 mL) in a high-pressure hydrogenation vessel. Pd/C 10% (0.019 g) was then added. The vesselwas installed in the reactor and, after 4 purges, the H2 pressure was set to 50 psi. After 2 h, the reaction was stopped and the mixturewas filtered on a Celite pad. The filtrate was concentrated invacuo and the crude product was purified by silica gel column chromatography (100% EtOAc), yielding 16 as a brownish solid ina 78% yield (0083 mmol, 0.015 g). 1H NMR (400 MHz, CD3OD): dH6.96 (1H, d, J = 1.7), 6.79 (1H, dd, J = 8.2, 1.6), 6.76 (1H, d, J = 8.1),4.60 (1H, t, J = 6.1), 3.85 (3H, s), 3.59 (2H, d, J = 6.2). 13C NMR(101 MHz, CD3OD): dC 148.8, 147.0, 134.8, 120.2, 115.9, 111.0,75.8, 68.8, 56.3. IR (NaCl): mmax 3341, 2933, 1604, 1518, 1274,1153, 1126, 1078, 1031, 877 cm1. HRMS (ESI-TOF, m/z): calcdfor C9H11O3 (M-H2O+H)+ = 167.0703, found 167.0713.
References:
Cardinal, Sébastien;Paquet-Côté, Pierre-Alexandre;Azelmat, Jabrane;Bouchard, Corinne;Grenier, Daniel;Voyer, Normand [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 7,p. 2043 - 2056]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

534-82-7
21 suppliers
$65.00/10mg

2426-87-1
242 suppliers
$8.00/5g

534-82-7
21 suppliers
$65.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

534-82-7
21 suppliers
$65.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

534-82-7
21 suppliers
$65.00/10mg