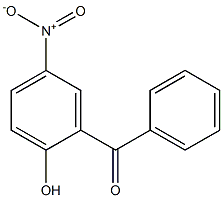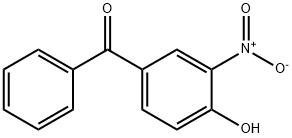
4-HYDROXY-3-NITROBENZOPHENONE synthesis
- Product Name:4-HYDROXY-3-NITROBENZOPHENONE
- CAS Number:5464-98-2
- Molecular formula:C13H9NO4
- Molecular Weight:243.21
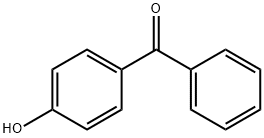
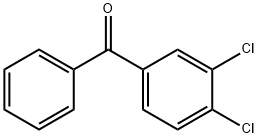
1137-42-4
515 suppliers
$6.00/25g

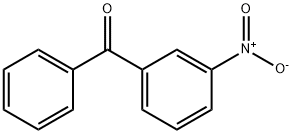
5464-98-2
19 suppliers
inquiry
Yield:5464-98-2 90%
Reaction Conditions:
with sodium nitrate;potassium hydrogensulfate;silica gel in dichloromethane at 20; for 12 h;
Steps:
General procedure for the synthesis of 2a and 2b
General procedure: To a solution of 4-hydroxybenzophenone or 4-hydroxyacetophenone (1.26 mmol) in dichloromethane(15 mL), potassium hydrogen sulfate (6.82 mmol), sodiumnitrate (7.32 mmol), and 0.875 g of wet silica 50% p/p wasadded. The slurry was was left under constant stirring atroom temperature until the completion of the reaction,checked by TLC (hexane/ethyl acetate, 8:2v/v). Then themixture was filtered through silica, the solid was washedwith dichloromethane and the solvent removed underreduced pressure. The products were then purified byrecrystallization. 4-hydroxy-3-nitrobenzophenone (2a) (Cohen et al. 1984)90% yield, yellow solid after recrystallization from water/methanol (1:1v/v); M.p. 105-107 °C; clogP = 3.12; IR(νmax cm-1): 3296, 3069, 1660, 1609, 1525, 1319. 1H NMR(300 MHz, CDCl3) δ: 7.28 (1H, d, H-5, 3J = 8.7 Hz),7.49-7.55 (2H, m, H-3′, H-5′), 7.64 (1H, tt, H-4′, 3J = 1.2Hz, 4J = 7.5 Hz), 7.74-7.78 (2H, m, H-2′, H-6′), 8.12 (1H,dd, H-6, 4J = 2.1 Hz, 3J = 8.7 Hz), 8.58 (1H, d, H-2, 4J =2.1 Hz). 13C NMR (75 MHz, CDCl3) δ: 193.2 (C, C-7),157.8 (C, C-4), 138.4 (CH, C-6, C, C-1′), 136.6 (C, C-3),132.9 (CH, C-4′), 129.8 (C, C-1), 129.6 (CH, C-2′, CH, C-6′), 128.6 (CH, C-3′, CH, C-5′), 127.8 (CH, C-2), 120.3(CH, C-5). pKa = 5.1.
References:
Folquitto, Laís R. S.;Nogueira, Priscila F.;Espuri, Patrícia F.;Gontijo, Vanessa S.;de Souza, Thiago B.;Marques, Marcos J.;Carvalho, Diogo T.;Júdice, Wagner A. S.;Dias, Danielle F. [Medicinal Chemistry Research,2017,vol. 26,# 6,p. 1149 - 1159]
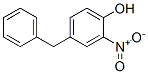
37021-63-9
8 suppliers
inquiry

5464-98-2
19 suppliers
inquiry
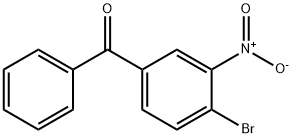
56107-03-0
2 suppliers
inquiry

5464-98-2
19 suppliers
inquiry