
4-HYDROXY-4-METHYL-PENT-2-YNOIC ACID synthesis
- Product Name:4-HYDROXY-4-METHYL-PENT-2-YNOIC ACID
- CAS Number:50624-25-4
- Molecular formula:C6H8O3
- Molecular Weight:128.13

67-64-1
6 suppliers
$19.10/10ml

471-25-0
226 suppliers
$10.00/1g

50624-25-4
16 suppliers
$130.00/100mg
Yield:50624-25-4 53%
Reaction Conditions:
with potassium hydroxide at 20;Inert atmosphere;
Steps:
4-Hydroxy-4-methyl-2-pentynoic acid (6) [CAS-No 50624-25-4]
Propiolic acid (1.2 mL, 19,5 mmol) was added to a mixture of KOH (6.56 g, 117 mmol) and acetone (20 mL) placed in a ice bath. After stirring overnight at rt, water was added untilthe solid dissolved. The resulting basic aqueous solutions was washed with Et2O (2 × 20mL) and then acified with 1 M HCl. The resulting acidic aqueous phase was extracted with EtOAc (3 × 30 mL) and the collected organic extracts were washed with HCl (1 M, 20 mL), brine (20 mL) and dried over MgSO4. Solvents were removed in vacuum resulting a brown oil. The product slowly crystallized after the addition of a 7:3 CH2Cl2/cyclohexane mixtureto afford 1.32 g (53% yield) of pale yellow crystals. Alternatively, the crude reaction mixture was purified by silica-gel chromatography (eluant pentane/Et2O in gradient from 8:2 to 5:5) to obtain 1.85 g (74% yield) as a colorless solid; mp 90-91 °C. 1H-NMR (300 MHz,benzene-d6): δ = 5.59 (s, br, 2 H), 1.30 (s, 6 H) ppm; 13C-NMR (75 MHz, CDC13): δ =154.0, 91.5, 73.5, 64.2, 30.1 ppm; IR (KBr): ν~= 3412 (s), 2989 (s), 2241 (m), 1703 (s),1372 (m), 1264 (s), 1164 (m), 941 (m) cm-1.
References:
Caporale, Andrea;Tartaggia, Stefano;Castellin, Andrea;De Lucchi, Ottorino [Beilstein Journal of Organic Chemistry,2014,vol. 10,p. 384 - 393] Location in patent:supporting information

124-38-9
121 suppliers
$214.00/14L
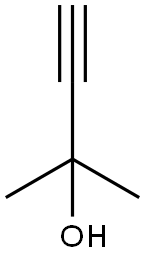
115-19-5
257 suppliers
$17.00/25mL

50624-25-4
16 suppliers
$130.00/100mg
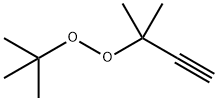
52258-96-5
0 suppliers
inquiry

50624-25-4
16 suppliers
$130.00/100mg
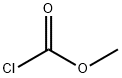
79-22-1
2 suppliers
$38.00/1000g

50624-25-4
16 suppliers
$130.00/100mg