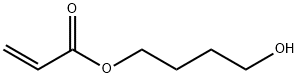
4-Hydroxybutyl acrylate synthesis
- Product Name:4-Hydroxybutyl acrylate
- CAS Number:2478-10-6
- Molecular formula:C7H12O3
- Molecular Weight:144.17
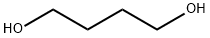
110-63-4
730 suppliers
$24.80/250g
292638-85-8
5 suppliers
inquiry

2478-10-6
186 suppliers
$5.00/25g
Yield:2478-10-6 98.3%
Reaction Conditions:
with 10H-phenothiazine;dioctyltin(IV) oxide in cyclohexane at 85; for 10 h;Concentration;Reagent/catalyst;
Steps:
III-4 Example III-4
[0440] The water layer obtained by the second extraction operation in Example III-1 was concentrated, to give 340 gof a mixture of 1,4-butanediol and dioctyltin oxide.[0441] Next, 340 g of the collected mixture was used in place of 1,4-butanediol used in Example III-1, and new 1,4-butanediol was added in the same amount as that of 1,4-butanediol used in Example III-1. At that time, since a dialkyltinoxide is contained in the mixture, a catalyst for transesterification was not additionally added.[0442] Thereafter, the reaction of 1,4-butanediol and methyl acrylate was carried out in the same manner as in ExampleIII-1 at a temperature of 85°C for 10 hours.[0443] After the completion of the reaction of 1,4-butanediol and methyl acrylate, the obtained reaction liquid washeated to 88°C under reduced pressure, and unreacted methyl acrylate was removed by distillation, to obtain 1080 g ofa reaction mixture.[0444] The components other than the solvent in the obtained reaction mixture were analyzed by gas chromatography.As a result, the content of 4-hydroxybutyl acrylate was 50.1% by mass, the content of 1,4-butanediol was 37.3% bymass, and the content of 1,4-butanediol diacrylate was 12.2% by mass in the components.[0445] As a first extraction operation, the obtained reaction mixture in an amount of 1088 g was supplied at a flow rateof 50 mL/h, and pure water was also supplied at a flow rate of 29.5 mL/h to the upper part of a pulsating type continuousextraction tower (filled tower with 25 mm diameter and 1.4 m length), cyclohexane was supplied over 22 hours at a flowrate of 87.8 mL/h to the lower part of the continuous extraction tower. The inside of the tower was heated to 30 to 35°C,and extraction was carried out continuously, to obtain a cyclohexane layer containing 1,4-butanediol diacrylate as a byproduct,and a water layer containing the resulting 4-hydroxybutyl acrylate and unreacted 1,4-butanediol.[0446] Next, the obtained water layer was dropwise added at a flow rate of 95 g/h to a column filled with 16 g of aweakly basic anion-exchange resin (trade name: Amberlite IRA 98, manufactured by Organo Corporation) to bring thewater layer into contact with the weakly basic anion-exchange resin.[0447] Next, as a second extraction operation, 1200 mL of the obtained water layer was supplied at a flow rate of 50mL/h to the upper part of the continuous extraction tower, and toluene as an extraction solvent was supplied over 24hours at a flow rate of 87.5 mL/h to the lower part of the continuous extraction tower. The inside of the tower was heatedto 35 to 40°C, and the continuous extraction was carried out, to obtain a toluene layer containing 4-hydroxybutyl acrylateand a water layer containing 1,4-butanediol. The content of 1,4-butanediol diacrylate in the components other thantoluene contained in the toluene layer was analyzed by gas chromatography. As a result, the content was found to be0.11% by mass.[0448] A 2 L three-necked glass flask was charged with the toluene layer in an amount of 1400 g obtained by thesecond extraction operation, and 0.008 g of hydroquinone monomethyl ether as a polymerization inhibitor was addedto the flask. The temperature in the bottom part of the flask was adjusted to 74 to 85°C, and toluene was removed underreduced pressure of 280 to 20 hPa by distillation for 14 hours, to concentrate 4-hydroxybutyl acrylate.[0449] The components contained in the condensed material were analyzed by gas chromatography. As a result,98.3% by mass of 4-hydroxybutyl acrylate, 0.11% by mass of 1,4-butanediol diacrylate and 1.2% by mass of 1,4-butanediol were contained in the components. The resulting condensed material was distilled by a thin film distillatory,and the content of an acid in the distillate was measured. As a result, the content of acrylic acid in the distillate was0.0015% by mass (15 ppm).[0450] From the above-mentioned results, it was found that 4-hydroxybutyl acrylate having a low content of an acidcan be efficiently collected even when the extraction operation is carried out by reusing the catalyst for transesterification.
References:
EP2860170,2015,A1 Location in patent:Paragraph 0440-0450
110-63-4
730 suppliers
$24.80/250g
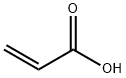
79-10-7
737 suppliers
$20.50/500ml

2478-10-6
186 suppliers
$5.00/25g
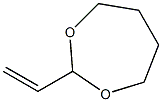
7434-86-8
0 suppliers
inquiry

2478-10-6
186 suppliers
$5.00/25g
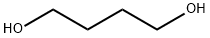
110-63-4
730 suppliers
$24.80/250g

814-68-6
396 suppliers
$21.21/5gm:

2478-10-6
186 suppliers
$5.00/25g
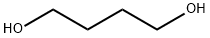
110-63-4
730 suppliers
$24.80/250g

292638-85-8
5 suppliers
inquiry

2478-10-6
186 suppliers
$5.00/25g
1070-70-8
242 suppliers
$5.00/5g