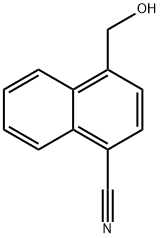
4-(HYDROXYMETHYL)NAPHTHALENE-1-CARBONITRILE synthesis
- Product Name:4-(HYDROXYMETHYL)NAPHTHALENE-1-CARBONITRILE
- CAS Number:79996-90-0
- Molecular formula:C12H9NO
- Molecular Weight:183.21

41014-20-4
24 suppliers
inquiry

79996-90-0
32 suppliers
inquiry
Yield:79996-90-0 85.3%
Reaction Conditions:
with lithium hydroxide monohydrate;anhydrous sodium carbonate in 1,4-dioxane at 100; for 3 h;
Steps:
68.B Step B: Synthesis of 4-(hydroxymethyl)-1-naphthalenenitrile
To a solution of 4-(bromomethyl)-1-naphthalenecarbonitrile (630 mg, 2.56 mmol) in 1,4-dioxane/water (6.5 mL/6.5 mL) was added sodium carbonate (542 mL) at room temperature mg, 5.11 mmol), heated to 100 degrees Celsius for 3 hours.The reaction was completed, cooled to room temperature, extracted with ethyl acetate (50 mL×L), washed, and the organic phase was washed with saturated brine (50 mL), then dried over anhydrous sodium sulfate, and finally concentrated under reduced pressure to obtain 400 mg 4-(hydroxymethyl)-1-naphthalenenitrile as a white solid (yield: 85.3%).
References:
WO2022/68772,2022,A1 Location in patent:Page/Page column 106-107
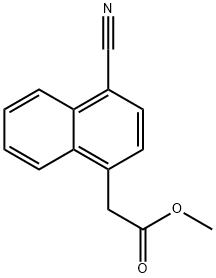
14311-32-1
4 suppliers
inquiry

79996-90-0
32 suppliers
inquiry
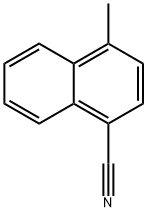
36062-93-8
56 suppliers
inquiry

79996-90-0
32 suppliers
inquiry

6627-78-7
107 suppliers
$13.00/1g

79996-90-0
32 suppliers
inquiry
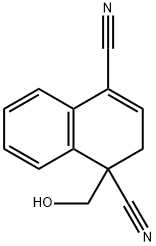
154463-61-3
0 suppliers
inquiry

79996-90-0
32 suppliers
inquiry