
4-(Hydroxymethyl)piperidine-1-carbaldehyde synthesis
- Product Name:4-(Hydroxymethyl)piperidine-1-carbaldehyde
- CAS Number:835633-50-6
- Molecular formula:C7H13NO2
- Molecular Weight:143.18
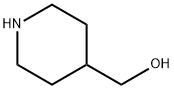
6457-49-4
382 suppliers
$18.00/25g
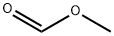
107-31-3
327 suppliers
$17.00/25mL

835633-50-6
31 suppliers
inquiry
Yield:835633-50-6 100%
Reaction Conditions:
Stage #1: 4-piperidinemethanol;Methyl formate at 0 - 20; for 2 h;
Stage #2: with sodium hydroxide
Stage #3: with hydrogenchloride in diethyl ether;dichloromethane;
Steps:
F
4-Piperidinemethanol (10 g, 87 mmol) was dissolved in methyl formate (7 mL, 113 mmol) 0 °C, and maintained at that temperature for 30 min, then allowed to reach 20 °C and stirred 90 min. Solid sodium hydroxide was added (0.87 g, pellets) and the mixture was left overnight. Dichloromethane was added, the NAOH removed by filtration and the solution treated with 1M HCL in ether (10 mL). The mixture was filtered through Celite and the solvent was removed under reduced pressure to afford the crude title COMPOUND. 1H NMR (400 MHz, CDC13) : 0. 85-1. 1 (2H, m); 1.55-1. 85 (3H, m); 2.5-2. 7 (1H, m); 2.95-3. 1 (1H, m); 3.3 (2H, D, J7 Hz); 3.6-3. 7 (1H, m); 4.1-4. 3 (1H, m); 8 (1H, s)
References:
WO2005/9443,2005,A1 Location in patent:Page/Page column 57

6457-49-4
382 suppliers
$18.00/25g

124-38-9
122 suppliers
$214.00/14L

835633-50-6
31 suppliers
inquiry