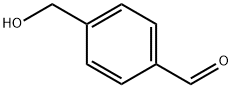
4-HYDROXYMETHYLBENZALDEHYDE synthesis
- Product Name:4-HYDROXYMETHYLBENZALDEHYDE
- CAS Number:52010-97-6
- Molecular formula:C8H8O2
- Molecular Weight:136.15
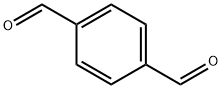
623-27-8
513 suppliers
$5.00/10g
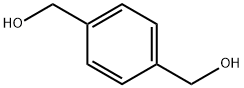
589-29-7
339 suppliers
$5.00/5g

52010-97-6
143 suppliers
$7.00/250mg
Yield:52010-97-6 83% ,589-29-7 17%
Reaction Conditions:
with C48H43ClN2P2Ru;ammonium formate in water;toluene at 90; for 9 h;Catalytic behavior;Schlenk technique;
Steps:
31 Example 31
Transfer hydrogenation in a biphasic system with formate salts as hydride donor on aldehyde substrates The selected aldehyde (2.5 mmol), HCOONH4 (10 mmol, 0.63 g) and complex (e.g. 1 .25 μηιοΙ, 1 mg; S/C = 2000) are transferred into a 50 ml Schlenk tube. Then toluene (1 .2 ml) and water (5 ml) are sequentially added. The biphasic mixture is subjected to four vacuum-argon cycles under vigorous stirring and then put into an oil bath at 90°C for the desired time. The reaction is sampled by removing ~1 ml of the mixture, diethyl ether (4 ml) is added, the organic phase separated, dried over MgS04, filtered and the solvent gently removed under reduced pressure. The crude residue was dissolved with CDCI3 and analyzed by H-NMR. Alternatively, the dried organic fraction is filtered over a short silica pad and the conversion determined by GC analysis. Table 11. TH of aldehydes catalyzed by complexes 13-15 with HC02NH4 in toluene/H20 at 90°C Aldehyde Complex S/C Substrate NH4-formate Time Alcohol By- molar molar;equivalents (h) (%) products (%L_ 13 5000 0.5 1 ; 2 16 60 0 13 5000 0.5 1 ; 2 22 76 0 14 5000 0.5 1 ; 2 15 96 0 14 5000 0.5 1 ; 2 24 97 0 14 5000 1 .0 1 ; 2 15 86 0 14 5000 1.0 1; 2 24 95 0 14 5000 0.5 1; 4 15 96 0 14 5000 0.5 1; 4 24 96 0 14 5000 1.0 1; 4 15 96 0 14 5000 1.0 1; 4 24 96 0 14 20000 2.0 2; 4 24 94 0 14 20000 2.0 2; 4 48 96 0 15 5000 0.5 1; 2 16 96 0 15 10000 2.0 1; 2 20 86 0 15 20000 2.0 1; 2 40 96 0 14 2000 0.5 1; 2 10 97 0 14 20000 2.0 2; 4 24 62 0 14 20000 2.0 2; 4 48 72 0 14 2000 0.5 2; 4 10 >99 0 14 2000 0.5 1; 2 3.5 >99 0 14 2000 0.5 1; 1.5 9 57: 83 0 58: 17 14 2000 0.5 1; 2 10 57: 71 0 58: 21 0 14 2000 0.5 1;4 10 57: 0 58: 99 14 2000 0.5 1; 2 10 97 54:10 14 5000 2.0 2; 4 16 78 0 14 5000 2.0 2; 4 24 86 0 14 5000 2.0 2; 4 48 97 0 14 5000 2.0 4; 4 16 84 0 14 5000 2.0 4; 4 24 91 0 14 5000 2.0 4; 4 48 94 0 14 10000 0.5 1;2 24 38 0 14 10000 0.5 1;2 38 49 0 With complexes 13-15 the transfer hydrogenation of aldehydes with NH4-formate is an improvement compared to using 2-propanol as hydride donor and K2C03 as base (examples 28 and 29). The use of less complex (higher S/C ratio) is possible and less by-products are formed. It is important to note that no primary amines are produced by reductive amination of the aldehyde. Interestingly, the presence of the toluene solvent as co-solvent is not entirely required, as shown in the table below. Toluene was not added to the reactions carried out on a 2.5 mmol substrate scale. Table 12. TH of aldehydes catalyzed by complex 14 with HC02NH4in H20 at 90°C Aldehyde Complex S/C Substrate NH4-formate Time Alcohol By- molar molar;equivalents (h) (%) products (%) 13 5000 0.5 1; 2 16 60 13 5000 0.5 1; 2 22 76 14 2000 2.5 mmol 1; 2 2 50 14 2000 2.5 mmol 1; 2 4 76 14 2000 2.5 mmol 1; 2 7 97 14 5000 2.5 mmol 1; 2 14 53 14 5000 2.5 mmol 1; 4 24 97 14 5000 2.5 mmol 2; 4 24 90 45 14 5000 0.5 1; 2 15 96 14 5000 0.5 1; 2 24 97 14 5000 1.0 1; 2 15 86 14 5000 1.0 1; 2 24 95 14 5000 0.5 1; 4 15 96 14 5000 0.5 1; 4 24 96 14 5000 1.0 1; 4 15 96 14 5000 1.0 1; 4 24 96 14 20000 2.0 2; 4 24 94 14 20000 2.0 2; 4 48 96 0 15 5000 0.5 1; 2 16 96 0 15 10000 2.0 1; 2 20 86 0 15 20000 2.0 1; 2 40 96 0 14 2000 2.5 mmol 1; 2 14 33 6 14 2000 2.5 mmol 1; 2 16 61 0 14 2000 0.5 1; 2 10 97 0 46 14 2000 2.0 2;4 11 97 0 14 10000 2.0 2;4 24 24 0 14 20000 2.0 2; 4 24 62 0 14 20000 2.0 2; 4 48 72 0 14 5000 2.0 2; 4 15 96 0 47 >99, 14 2000 0.5 2; 4 10 0 48 65[bl 14 2000 0.5 1; 2 3.5 >99 0 55 14 2000 2.0 2;4 3.5 99, 65[bl 0 14 2000 0.5 1; 1.5 9 57: 83 0 56 58: 17 14 2000 0.5 1; 2 10 57: 71 0 58: 21 14 2000 0.5 1;4 10 57: 0 0 58: 99 14 2000 0.5 1; 2 10 97 54:10 14 5000 2.0 2; 4 16 78 54:6 14 5000 2.0 2; 4 24 86 0 52 14 5000 2.0 2; 4 48 97 54:12 14 5000 2.0 4; 4 16 84 0 14 5000 2.0 4; 4 24 92 54:5 14 5000 2.0 4; 4 48 94 54:7 14 10000 0.5 1 ; 2 24 38 14 10000 0.5 1 ; 2 38 49 59 14 10000 2.0 4; 4 20 98 0 60 14 10000 2.0 2; 4 24 65 0 60 14 10000 2.0 4; 4 24 97 0 61 14 5000 2.0 4;4 20 98, 88[bl 62 14 5000 2.0 2;4 8 95 63 14 2000 2.0 4;4 9 96, 79[bl Conversion and product content were determined by GC analysis or by -NMR spectroscopy. Isolated yield. On 2.5 mmol scale, reduction of benzaldehyde 45 (0.5 molar in toluene) at S/C =2000, 90°C and 4 equivalents of 2M aqueous Na-formate gave only traces of benzylalcohol after 14 hours. Use of 4 equivalents of (NEt3H)-formate improves the yield to 50 % in 22 hours. Use of 5 equivalents of (NEt3H)-formate on frans-cinnamaldehyde 52 gives after 18 hours 80 % of allylic alcohol 53 and 15 % of saturated alcohol 54. NH4-formate is preferred over the other formate reagents.
References:
JOHNSON MATTHEY PUBLIC LIMITED COMPANY;BALDINO, Salvatore;BARATTA, Walter;BLACKABY, Andrew;BRYAN, Richard, Charles;FACCHETTI, Sarah;JURCIK, Vaclav;NEDDEN, Hans, Guenter WO2016/193761, 2016, A1 Location in patent:Page/Page column 85-87

623-27-8
513 suppliers
$5.00/10g

52010-97-6
143 suppliers
$7.00/250mg

589-29-7
339 suppliers
$5.00/5g

52010-97-6
143 suppliers
$7.00/250mg

1571-08-0
320 suppliers
$10.00/5g

52010-97-6
143 suppliers
$7.00/250mg
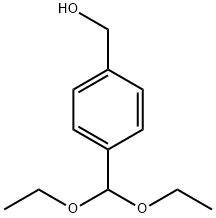
125734-44-3
5 suppliers
inquiry

52010-97-6
143 suppliers
$7.00/250mg