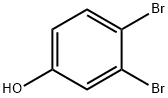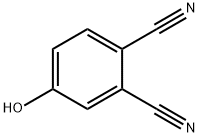
4-Hydroxyphthalonitrile synthesis
- Product Name:4-Hydroxyphthalonitrile
- CAS Number:30757-50-7
- Molecular formula:C8H4N2O
- Molecular Weight:144.13
31643-49-9
439 suppliers
$6.00/5g

30757-50-7
125 suppliers
$60.00/100mg
23277-29-4
4 suppliers
inquiry
Yield: 0.098 g , 2.451 g
Reaction Conditions:
with potassium carbonate;3-Hydroxypropionitrile in N,N-dimethyl-formamide at 40; for 99 h;Inert atmosphere;Molecular sieve;
Steps:
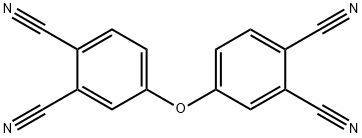
Synthesis process of 4,40-oxydiphthalonitrile (3)
3-hydroxypropanenitrile (6.3 g, 88.63 mmol) was dissolved indry DMF (125 mL) under nitrogen and 4-nitrophthalonitrile(5.08 g, 29.36 mmol) and molecular sieve 4Å (10.80 g) wereadded. After stirring for 2 h, finely ground anhydrous potassiumcarbonate (13.50 g, 97.831 mmol) was added in portionsduring 3 h with efficient stirring. The reaction mixturewas stirred at 40 C under nitrogen for 96 h. The mixturewas then poured in to ice-water (300 g). The precipitate wasfiltered off, washed with water until neutral and dried. Thecrude product was extracted with chloroform (450 mL).The combined organic extracts were washed with water anddried with anhydrous Na2SO4 and evaporated to dryness.The resulting product was washed with water and thendried, yield 4,40-oxydiphthalonitrile (3) 0,098 g. The singlecrystals were obtained in DMF at room temperature viaslow evaporation. The other filtrate was acidified to pH 3with HCl and filtered off and washed with water until neutraland then dried, yield 4-hydroxyphthalonitrile (4)2.451 g. It was not necessary to purify the product more.The spectral specifications of 4,4’-oxydiphthalonitrile (3)and 4-hydroxyphthalonitrile (4) were consistent with literaturevalues.[27-32]
References:
Eryılmaz, Serpil;Akdemir, Nesuhi;İnkaya, Ersin [Spectroscopy Letters,2019,vol. 52,# 1,p. 28 - 42]

31643-49-9
439 suppliers
$6.00/5g

30757-50-7
125 suppliers
$60.00/100mg
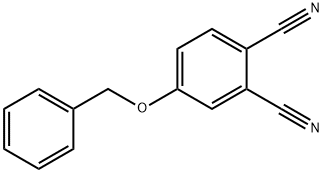
86312-75-6
1 suppliers
inquiry

30757-50-7
125 suppliers
$60.00/100mg
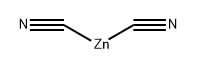
557-21-1
99 suppliers
$12.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

30757-50-7
125 suppliers
$60.00/100mg