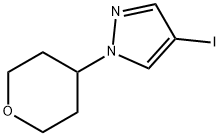
4-iodo-1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazole synthesis
- Product Name:4-iodo-1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazole
- CAS Number:1175274-93-7
- Molecular formula:C8H11IN2O
- Molecular Weight:278.09

3469-69-0
401 suppliers
$5.00/1g
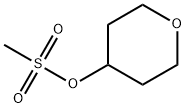
134419-59-3
111 suppliers
$6.00/1g

1175274-93-7
25 suppliers
inquiry
Yield:1175274-93-7 410 mg
Reaction Conditions:
Stage #1: 4-iodopyrazolewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 1 h;
Stage #2: 4-oxanyl methanesulfonate in N,N-dimethyl-formamide;mineral oil at 0 - 100; for 24 h;
Steps:
23.1 Step 1) 4-iodo-l-(tetrahydro-2H-pyran-4-yl)-lH-pyrazole
To a solution of 4-iodo-lH-pyrazole (388 mg, 2.0 mmol) in dry DMF (20 mL) was added NaH (90 mg, 3.0 mmol, 80% NaH/mineral oil) slowly at 0 °C. The mixture was stirred at 0 °C for 1 hour, then a solution of the crude product above (396 mg, 2.2 mmol) in DMF (5 mL) was added dropwise at 0 °C. The mixture was heated to 100 °C and stirred further for 24 hours, then cooled to rt, quenched with water (0.5 mL), and concentrated in vacuo. The residue was suspended in DCM (100 mL) and washed with water (50 mL). The separated organic layer was dried over anhydrous Na2S04, and concentrated in vacuo to give the title compound as a white solid (410 mg, 74%). MS (ESI, pos. ion) m/z: 279.0 (M +1); NMR (400 MHz, CDC ): δ 7.52 (s, 1H), 7.48 (s, 1H), 4.42-4.30 (m, 1H), 4.15-4.05 (m, 2H), 3.58-3.47 (m, 2H), 2.12-1.98 (m, 4H).
References:
WO2014/193647,2014,A2 Location in patent:Paragraph 0257
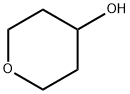
2081-44-9
295 suppliers
$6.00/1g

1175274-93-7
25 suppliers
inquiry