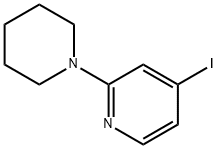
4-Iodo-2-(1-piperidinyl)pyridine synthesis
- Product Name:4-Iodo-2-(1-piperidinyl)pyridine
- CAS Number:1239969-35-7
- Molecular formula:C10H13IN2
- Molecular Weight:288.13

110-89-4
3 suppliers
$15.96/100ML
22282-70-8
268 suppliers
$8.00/1g
1209468-41-6
0 suppliers
inquiry

1239969-35-7
9 suppliers
inquiry
Yield:1239969-35-7 42%
Reaction Conditions:
with palladium diacetate;potassium carbonate;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 130; for 16 h;Inert atmosphere;Sealed vial;Buchwald-Hartwig amination;
Steps:
4.2. General procedure A
General procedure: Dihalopyridine (1 equiv), amine (1.2 equiv), K2CO3 (3.5 equiv), Pd(OAc)2 (2 mol %), and BINAP (2 mol %) were charged into a microwave vial and dry toluene was added. The vial was then sealed, evacuated, and flushed with argon. Then the reaction mixture was irradiated at 180 °C in a CEM Explorer microwave unit for 30 min (in few cases 45 min) with stirring. After cooling to rt, the solid material was removed by filtration and washed with 10 mL of EtOAc or CH2Cl2. The organic phases were combined and the solvent was evaporated. The resulting crude product was purified by flash column chromatography.
References:
Koley, Moumita;Schnürch, Michael;Mihovilovic, Marko D. [Tetrahedron,2011,vol. 67,# 23,p. 4169 - 4178] Location in patent:supporting information; experimental part

110-89-4
3 suppliers
$15.96/100ML

22282-70-8
268 suppliers
$8.00/1g

1239969-35-7
9 suppliers
inquiry