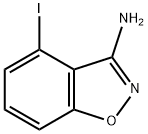
4-Iodo-benzo[d]isoxazol-3-ylaMine synthesis
- Product Name:4-Iodo-benzo[d]isoxazol-3-ylaMine
- CAS Number:1012367-55-3
- Molecular formula:C7H5IN2O
- Molecular Weight:260.03
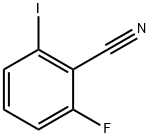
79544-29-9
187 suppliers
$10.00/5g
![4-Iodo-benzo[d]isoxazol-3-ylaMine](/CAS/20150408/GIF/1012367-55-3.gif)
1012367-55-3
16 suppliers
inquiry
Yield:1012367-55-3 69%
Reaction Conditions:
with acetylhydroxamic acid;potassium tert-butylate in N,N-dimethyl-formamide at 20; for 12.5 h;
Steps:
41.1 Step 1: 4-iodobenzo[d]isoxazol-3-amine
Step 1: 4-iodobenzo[d]isoxazol-3-amine 61 mg of acetohydroxamic acid was dissloved in 1 ml of N,N-dimethylformamideand 91 mg of potassium t-butoxide was added slowly with stirring and stirred for 0.5 h at room temperature. Then 100mg of 2-fluoro-6-iodobenzonitrile was added slowly and stirred for 12 hours at room temperature. Then 10 ml of waterwas added and the mixture was filtered. The filter cake was washed with water and dried to give 72 mg of 4-iodobenzo[d]isoxazol-3-amine as white solid. Yield: 69%.1H NMR (300 MHz, DMSO-d6) δ (ppm): 5.93 (s, 2H), 7.24-7.29 (m, 1H), 7.55 (dd, J= 8.4, 0.6 Hz, 1H), 7.69-7.71 (m, 1H).
References:
EP3112351,2017,A1 Location in patent:Paragraph 0163