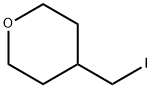
4-(Iodomethyl)tetrahydro-2H-pyran synthesis
- Product Name: 4-(Iodomethyl)tetrahydro-2H-pyran
- CAS Number:101691-94-5
- Molecular formula:C6H11IO
- Molecular Weight:226.06
Preparation: 4-Iodomethyltetrahydropyran; A mixture of Preparation (328g, 1.69mol) and sodium iodide (507g, 3.4mol) in acetone (3.3L) was refluxed for 4h. TLC (diethyl ether) showed significant mesylate remaining so further sodium iodide (127g, 0.65mol) was added and reflux continued for 16h. The mixture was cooled and filtered. The resulting cake was washed with acetone, dried, and11then partitioned between diethyl ether (800mL) and water (800mL). The aqueous phase was re-extracted with diethyl ether (200mL), the ether extracts combined and washed with 10% sodium thiosulphate solution (300mL) which decolourised the extract. Final washing with water (300mL), drying (MgS04) and then removal of the solvent provided the 4-(IODOMETHYL)TETRAHYDRO-2H-PYRAN (365g, 92% yield).
101691-65-0
53 suppliers
$17.00/250mg

101691-94-5
113 suppliers
$22.00/0.25 / G
Yield: 94%
Reaction Conditions:
with sodium iodide in acetone for 4 h;Reflux;
Steps:
44.2
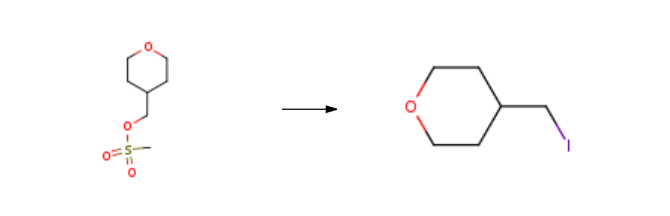
Step 2 [0340] (Tetrahydro-2H-pyran-4-yl)methyl 4-methylbenzenesulfonate obtained in Step 1 (1.20 g, 4.44 mmol) was dissolved in acetone (15 mL), sodium iodide (2.00 g, 13.3 mmol) was added thereto, and under reflux with heating, the mixture was stirred for 4 hours. After cooling the reaction mixture to room temperature, the precipitated solid was removed by filtration, and the filtrate was evaporated under reduced pressure. Chloroform was added to the residue, and the precipitated solid was removed by filtration. The filtrate was concentrated under reduced pressure, whereby 4-(iodomethyl)tetrahydro-2H-pyran (0.946 g, 94%) was obtained. 1H NMR (300 MHz, CDCl3, δ): 3.99-3.96 (m, 2H), 3.37 (td, J = 11.7, 2.1 Hz, 2H), 3.10 (d, J = 6.6 Hz, 2H), 1.81-1.65 (m, 3H), 1.37-1.24 (m, 2H).
References:
Kyowa Hakko Kirin Co., Ltd.;FURUTA, Takayuki;SAWADA, Takashi;DANJO, Tomohiro;NAKAJIMA, Takahiro;UESAKA, Noriaki EP2881394, 2015, A1 Location in patent:Paragraph 0340

132291-95-3
31 suppliers
$45.00/50mg

101691-94-5
113 suppliers
$22.00/0.25 / G
14774-37-9
246 suppliers
$5.00/1g

101691-94-5
113 suppliers
$22.00/0.25 / G

132291-95-3
31 suppliers
$45.00/50mg

101691-94-5
113 suppliers
$22.00/0.25 / G