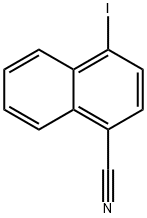
4-Iodonaphthalene-1-carbonitrile synthesis
- Product Name:4-Iodonaphthalene-1-carbonitrile
- CAS Number:140456-96-8
- Molecular formula:C11H6IN
- Molecular Weight:279.08
58728-64-6
71 suppliers
$78.00/100mg

140456-96-8
5 suppliers
inquiry
Yield:140456-96-8 67%
Reaction Conditions:
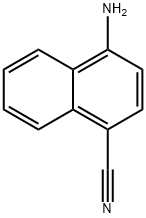
Stage #1: 4-amino-1-naphthonitrilewith hydrogenchloride;acetic acid;sodium nitrite in water at 0; for 2 h;
Stage #2: with iodine;potassium iodide in water at 0; for 2 h;
Steps:
169.B
Step B. 4-Iodo-l-naphthonitrile; A solution of NaN02 (0.83 g, 12.1 mmol) in water (10 mL) was added over 30 min. to a mixture of 4-amino-1-naphtonitrile (1.94 g, 11.5 mmol), concentrated HCI (12 mL) and glacial acetic acid (25 mL) at 0°C. The reaction mixture was stirred for 1.5 hr. and cold water (25 mL) was added. A solution of KI (2.29 g, 13.8 mmol) and iodine (1.75 g, 6.9 mmol) in water (15 mL) was added. The reaction mixture was stirred for 2 hrs. at 0°C and allowed to warm to ambient temperature. The product was extracted with EtOAc, washed with water and brine, and dried over anhydrous Na2S04. The solvent was concentrated and the product was purified on silica gel by flash chromatography to provide the title compound as white solid (2,21 g, 67 %).
References:
WO2005/115986,2005,A1 Location in patent:Page/Page column 183