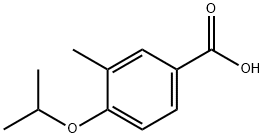
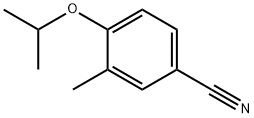
4-Isopropoxy-3-Methylbenzoic acid synthesis
- Product Name:4-Isopropoxy-3-Methylbenzoic acid
- CAS Number:856165-81-6
- Molecular formula:C11H14O3
- Molecular Weight:194.23
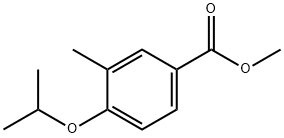
864178-56-3
4 suppliers
inquiry

856165-81-6
19 suppliers
inquiry
Yield:856165-81-6 82%
Reaction Conditions:
Stage #1: methyl 3-methyl-4-isopropoxybenzoatewith water;lithium hydroxide in tetrahydrofuran at 65; for 6 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; pH=2;
Steps:
Lithium hydroxide (4.4 g, 181.6 mmol) was added to a solution of methyl 4-isopropoxy-3 -methylbenzoate (11.2 g, 53.8 mmol) in tetrahydrofuran (31 mL) and water (31 mL). The mixture was rapidly stirred and heated at 65 °C for 6 hours. The reaction mixture was cooled, diluted with water (75 mL) and extracted with ether (2 x 50 mL). The aqueous layer was acidified to pH 2 with 6N aq. HC1 and extracted with ethyl acetate (4 x 75 mL). The combined organics were washed with water (75 mL) and brine solution (75 mL) and layers were separated. The organics were dried over MgS04 and concentrated in vacuo to give 4-isopropoxy- 3-methyl-benzoic acid (9.5 g, 82 %) as colorless crystals. ESI-MS m/z calc. 194.2, found 195.3 (M+l)+; Retention time: 1.53 minutes (3 min run). lH NMR (400 MHz,DMSO) δ 7.78 - 7.71 (m, 2H), 7.02 (d, J = 8.6 Hz, 1H), 4.70 (dt, J = 12.1 , 6.0 Hz, 1H), 2.15 (s, 3H), 1.30 (d, J = 6.0 Hz, 6H).
References:
WO2012/125613,2012,A1 Location in patent:Page/Page column 147

499-76-3
205 suppliers
$6.00/1g

856165-81-6
19 suppliers
inquiry
42113-13-3
95 suppliers
$14.00/1g

856165-81-6
19 suppliers
inquiry
185147-08-4
188 suppliers
$7.00/5g

856165-81-6
19 suppliers
inquiry
610797-50-7
4 suppliers
inquiry

856165-81-6
19 suppliers
inquiry