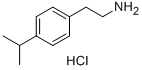
4-ISOPROPYLPHENETHYLAMINE HYDROCHLORIDE synthesis
- Product Name:4-ISOPROPYLPHENETHYLAMINE HYDROCHLORIDE
- CAS Number:61035-87-8
- Molecular formula:C11H18ClN
- Molecular Weight:199.7203
4395-87-3
91 suppliers
$15.00/1g

61035-87-8
19 suppliers
$89.59/1g
Yield:61035-87-8 35%
Reaction Conditions:
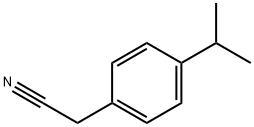
Stage #1: (4-isopropylphenyl)acetonitrilewith lithium aluminium tetrahydride in diethyl ether at 0; for 1 h;Heating / reflux;
Stage #2: with water;ammonium chloride in diethyl ether at 0;
Stage #3: with hydrogenchloride in methanol at 20; for 0.583333 h;
Steps:
120
Intermediate 120; (2-[4-(I -methylethyl)phenyllethyl)amine hydrochloride; A solution of LiAIH4 (838 mg, 20.97 mmol) in diethyl ether (15 ml) was cooled at 00C under argon. [4-(1-methylethyl)phenyl]acetonitrile (Intermediate 119) (1.67 g, 10.48 mmol) in solution of diethyl ether (10 ml) was added drop by drop at 00C. The mixture was stirred at reflux for 1 hour. The mixture was cooled at 0°C, NH4CI (1.246 g) was added and 1.2 ml of water was added. Diethyl ether was added and the mixture was dried overMgSO4 and filtered on celite and washed with diethyl ether. The filtrate was evaporated.
References:
WO2009/47240,2009,A1 Location in patent:Page/Page column 84-85