
4-MERCAPTOCINNAMIC ACID synthesis
- Product Name:4-MERCAPTOCINNAMIC ACID
- CAS Number:28995-22-4
- Molecular formula:C9H8O2S
- Molecular Weight:180.22
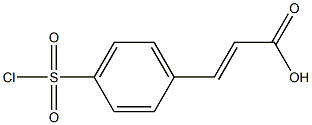
155378-73-7
2 suppliers
inquiry

64-17-5
715 suppliers
$10.00/50g

28995-22-4
19 suppliers
$130.00/100mg
Yield:28995-22-4 60%
Reaction Conditions:
with stannous chloride in hydrogenchloride;sodium hydroxide;water;
Steps:
1-2:
Synthesis of Trans-(4-Mercapto)Cinnamic Acid A 2,000 ml three-necked flask equipped with a stirrer, a nitrogen gas introduction pipe and a reflux condenser was charged with 700 ml of ethyl alcohol, and then 51.2 g of trans-(4-chlorosulfonyl)cinnamic acid synthesised in Example 1-1 above, and nitrogen gas was introduced in the flask for 5 minutes to purge the flask with nitrogen. Then, while stirring the solution, 250 ml of concentrated hydrochloric acid and then 200 g of anhydrous stannous chloride were added. After completion of the addition, the reaction mixture was heated under reflux for 30 minutes. Then, the reaction mixture was poured in an aqueous hydrochloric acid solution (concentrated hydrochloric acid:cold water=1:1 (v/v)), and the solution obtained was filtered to obtain yellow solids, which were washed with 100 ml of concentrated hydrochloric acid and then 100 ml of water several times, respectively. The crude product thus obtained was dissolved in 300 ml of an aqueous 10% sodium hydroxide solution, and stirred for 3 minutes, followed by filtering insoluble matter. Hydrochloric acid was added to the aqueous alkali solution to render it litmus acidic to precipitate crystals, which were filtered. The crystals thus obtained were washed with water and then with hexane several times, respectively, and then dried under vacuum to obtain 22.5 g of yellow powder (yield: 60%, melting point: 217° C.). Note that the above-mentioned procedure was conducted in a nitrogen atmosphere.
References:
US5308536,1994,A

155378-73-7
2 suppliers
inquiry

28995-22-4
19 suppliers
$130.00/100mg