
4-METHOXY-3'-TRIFLUOROMETHYLBIPHENYL synthesis
- Product Name:4-METHOXY-3'-TRIFLUOROMETHYLBIPHENYL
- CAS Number:194873-98-8
- Molecular formula:C14H11F3O
- Molecular Weight:252.23
Yield:194873-98-8 96%
Reaction Conditions:
with potassium phosphate tribasic trihydrate;cis-[PdCl2(Ph2PNHpy)] in N,N-dimethyl-formamide at 100; for 15 h;Schlenk technique;Suzuki-Miyaura Coupling;
Steps:
1 4.4. General procedure for the Suzuki-Miyaura cross-coupling reaction of aryl chlorides with aryl boronic acids
General procedure: In a 25 mL Schlenk tube containing a magnetic stirrer bar, 4-chloroacetophenone (2a) (155 mg, 1 mmol) was mixed with 4-methoxyphenylboronic acid (3a) (228 mg, 1.5 mmol). Then trihydrate potassium phosphate (798 mg, 3 mmol) and DMF (5 mL) were added. The dissolved mixture of 1 (1.50 mL, 0.015 mmol) was transferred into the tube by a pipette. The tube was placed in a 100°C oil bath and stirred for 15 h. At the end of the reaction, the mixture was cooled to room temperature, added with water (5 mL), and then extracted with ethyl acetate (3×5 mL). The organic layer was dried over anhydrous Na2SO4. After evaporation of the solvent, the residue was subjected to silica gel column chromatography to give the cross-coupling product.
References:
Xu, Lin-Yan;Liu, Chun-Yu;Liu, Shi-Yuan;Ren, Zhi-Gang;Young, David James;Lang, Jian-Ping [Tetrahedron,2017,vol. 73,# 22,p. 3125 - 3132]
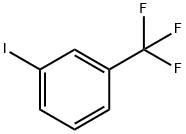
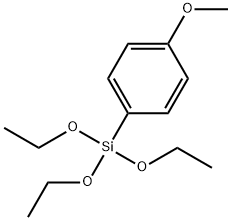
401-81-0
237 suppliers
$10.00/10g
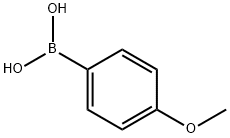
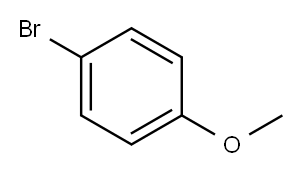
5720-07-0
456 suppliers
$6.00/5g

194873-98-8
10 suppliers
$437.00/1g
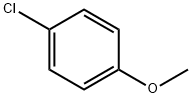
623-12-1
278 suppliers
$10.00/25g
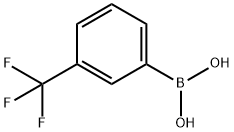
1423-26-3
340 suppliers
$6.00/1g

194873-98-8
10 suppliers
$437.00/1g


![Lithium, [3-(trifluoromethyl)phenyl]-](/CAS/20211123/GIF/368-49-0.gif)