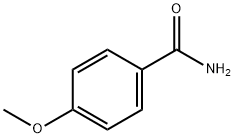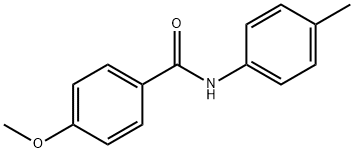
4-Methoxy-N-(4-Methylphenyl)benzaMide, 97% synthesis
- Product Name:4-Methoxy-N-(4-Methylphenyl)benzaMide, 97%
- CAS Number:39192-94-4
- Molecular formula:C15H15NO2
- Molecular Weight:241.29
Yield:39192-94-4 89%
Reaction Conditions:
with N-[2-(4-sulphonatephenylamino)-4-methoxy-1,3,5-triazin-6-yl]-4-methylmorpholinium inner salt in N,N-dimethyl-formamide; for 24 h;Product distribution / selectivity;
Steps:
25
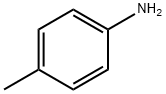
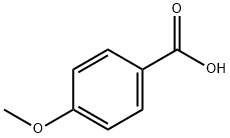
Example 25. In a flask, p-methoxybenzoic acid (0.152 g, 0.001 mol), p-toluidine (0.107 g, 0.001 mol), SPMT x 5H2O (0.471 g, 0.001 mol) were dissolved in DMF (2 ml) and stirred using a magnetic stirrer. After 24 hours, a mixture of 25 ml of 1 N solution of NaHCO3 and 25 ml of saturated sodium chloride solution were added to the reaction solution. The precipitate was filtered off, washed with 25 ml of 1 N hydrochloric acid, 25 ml of water, and dried in the air to a constant weight. 4-Methoxy-N-[4-methylphenyl]-benzamide (0.215 g, 89% yield) was obtained in the form of a white powder, having the melting point of 150-152°C (lit. 156-157°C). The results of NMR analyses of the obtained compound are as follows: 1H NMR (CDCl3): δ = 2.332 (s, CAr-CH3, 3 H); 3.860 (s, O-CH3, 3 H); 6.949 (d, JH-H = 8.75 Hz, CHAr, 2 H); 7.154 (d, JH-H = 8.5 Hz, CHAr, 2 H); 7.504 (d, JH-H = 8.5 Hz, CHAr, 2 H); 7.762 (s, NH, 1 H); 7.827 (d, JH-H = 8.75 Hz, CHA, 2 H);
References:
EP2407460,2012,A1 Location in patent:Page/Page column 10