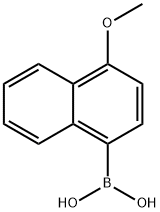
4-METHOXYNAPHTHALENE-1-BORONIC ACID synthesis
- Product Name:4-METHOXYNAPHTHALENE-1-BORONIC ACID
- CAS Number:219834-95-4
- Molecular formula:C11H11BO3
- Molecular Weight:202.01
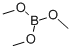
121-43-7
345 suppliers
$13.00/25mL
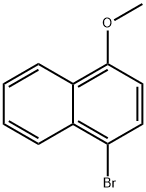
5467-58-3
127 suppliers
$9.00/1g

7732-18-5
463 suppliers
$12.69/100ml

219834-95-4
73 suppliers
$56.00/1g
Yield:219834-95-4 61%
Reaction Conditions:
Stage #1:1-bromo-4-methoxynaphthalene with n-butyllithium in tetrahydrofuran;hexane at -78 - 0; for 1 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran;hexane at 20; for 24 h;Inert atmosphere;
Stage #3:water with hydrogenchloride for 1 h;Inert atmosphere;
Steps:
Synthesis of 4-methoxynaphthalen- 1-ylboronic acid
An excess of 1.6 M n-BuLi in hexane(162 ml, 258 mmol) was added to a solution of 1-bromo-4-methoxynaphthalene(50.9 g, 215 mmol) in 800 ml dry tetrahydrofuran at -78° C. under N2. The reaction mixture was then maintained at 0° C. for 1 h before cooling to -78° C. Trimethylborate(35 g, 335 mmol) was added dropwise; the solution was then warmed slowly to room temperature and stirred for 24 h. 2N HCl (250 ml)was added and then the mixture was stirred for a further 1 h. The reaction mixture was extracted with ethyl acetate and water, dried with anhydrous magnesium sulfate, the solvent was evaporated in vacuo, and the residue was crystallized(n-hexane) to give the 4-methoxynaphthalen-1-ylboronic acid (31.8 g, 157 mmol, 61%) as a white solids.
References:
LUMINESCENCE TECHNOLOGY CORPORATION;Yen, Feng-Wen US9537103, 2017, B1 Location in patent:Page/Page column 53

5467-58-3
127 suppliers
$9.00/1g

219834-95-4
73 suppliers
$56.00/1g

5419-55-6
370 suppliers
$12.00/5g

5467-58-3
127 suppliers
$9.00/1g

7732-18-5
463 suppliers
$12.69/100ml

219834-95-4
73 suppliers
$56.00/1g
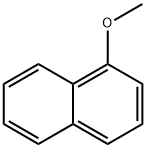
2216-69-5
255 suppliers
$5.00/10g

219834-95-4
73 suppliers
$56.00/1g