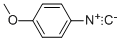
4-METHOXYPHENYL ISOCYANIDE synthesis
- Product Name:4-METHOXYPHENYL ISOCYANIDE
- CAS Number:10349-38-9
- Molecular formula:C8H7NO
- Molecular Weight:133.15
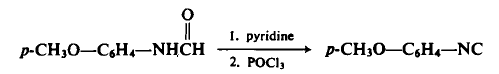
104-94-9
475 suppliers
$9.00/1g
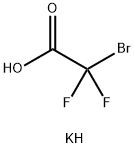
87189-16-0
69 suppliers
$14.00/250mg

10349-38-9
56 suppliers
$31.00/100mg
Yield: 68%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100; for 12 h;Inert atmosphere;
Steps:
13
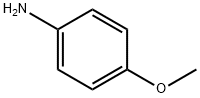
Put sodium difluorochloroacetate 1 (0.2mmol, 31mg), K2CO3 (0.2mmol, 28mg) and p-anisidine (2a, 0.1mmol, 12.3mg) into a 25mL reaction tube, evacuated and filled with nitrogen (three times ). DMF (1 mL) was injected with a syringe under nitrogen protection. Stir in an oil bath at 100°C for 12 hours. After cooling to room temperature, filter and add the reaction solution and 20 mL of water to the extraction funnel, then add 25 mL of dichloromethane for extraction, and then take the organic layer and add a small amount of silica gel to spin dry column chromatography. Yellow solid product 3a (70% yield).
References:
CN112279789, 2021, A Location in patent:Paragraph 0033; 0088-0089

5470-34-8
53 suppliers
$36.20/1g

10349-38-9
56 suppliers
$31.00/100mg
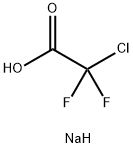
1895-39-2
284 suppliers
$6.00/5g

104-94-9
475 suppliers
$9.00/1g

10349-38-9
56 suppliers
$31.00/100mg

104-94-9
475 suppliers
$9.00/1g

10349-38-9
56 suppliers
$31.00/100mg
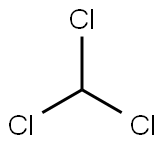
67-66-3
6 suppliers
$33.60/500ml

104-94-9
475 suppliers
$9.00/1g

10349-38-9
56 suppliers
$31.00/100mg