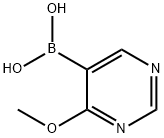
4-Methoxypyrimidin-5-ylboronicacid synthesis
- Product Name:4-Methoxypyrimidin-5-ylboronicacid
- CAS Number:909187-37-7
- Molecular formula:C5H7BN2O3
- Molecular Weight:153.93
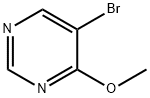
4319-85-1
64 suppliers
$28.00/100mg

909187-37-7
32 suppliers
inquiry
Yield:-
Reaction Conditions:
with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexane at -70 - -20; for 0.5 h;
Steps:
87.3
To a solution of 5-bromo-4-methoxypyrimidine (0.460 g, 2.43 mmol) and triisopropyl borate (0.671 mL, 2.92 mmol) in 12 mL of THF at -70° C. was added butyllithium (1.6 m in hexanes, 1.83 mL, 2.92 mmol) dropwise. The addition took 15 minutes and the reaction turned light yellow. The reaction stirred for 30 minutes at -70° C. and was then allowed to warm up to -20° C. The reaction was quenched with ammonium chloride (sat.) and extracted with ethyl acetate. The aqueous layer was concentrated and NaCl (sat.) was added. The aqueous layer was extracted with 3/2 chloroform/iPrOH (3×). The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated to give 166 mg of 4-methoxypyrimidin-5-ylboronic acid as a light yellow solid. ES+=155.2 (M+H)
References:
Amgen Inc. US2006/199817, 2006, A1 Location in patent:Page/Page column 49