
4-METHYL-1-(TRIFLUOROACETYL)-3-THIOSEMICARBAZIDE synthesis
- Product Name:4-METHYL-1-(TRIFLUOROACETYL)-3-THIOSEMICARBAZIDE
- CAS Number:25366-21-6
- Molecular formula:C4H6F3N3OS
- Molecular Weight:201.17
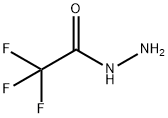
1538-08-5
119 suppliers
$5.00/100mg
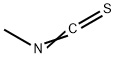
556-61-6
175 suppliers
$27.50/5g

25366-21-6
10 suppliers
inquiry
Yield:-
Reaction Conditions:
in ethanol at 80;
Steps:
General procedure: The requisite acid hydrazide (1.0 equiv) was dissolved or suspended in ethanol (1.5mL/mmol) at r.t. The appropriate isothiocyanate (1.0 equiv) was added in one portion. The reaction flask was immersed in an oil bath and stirred at 80°C until the reaction was completed (2-3h, TLC: DCE/MeOH 5:1). After cooling to room temperature, the product precipitated, which was filtered off, washed with cold ethanol (1mL/mmol) and dried thoroughly under reduced pressure. The product was TLC pure (average yield: 65-85%).
References:
Frackenpohl, Jens;Schneider, Linn;Decker, Luka J.B.;Dittgen, Jan;Fenkl, Franz;Fischer, Christian;Franke, Jana;Freigang, Joerg;Getachew, Rahel;Gonzalez Fernandez-Nino, Susana M.;Helmke, Hendrik;Hills, Martin J.;Hohmann, Sabine;Kleemann, Jochen;Kurowski, Karoline;Lange, Gudrun;Luemmen, Peter;Meyering, Nicole;Poree, Fabien;Schmutzler, Dirk;Wrede, Sebastian [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 24,art. no. 115142]