
4-Methyl-1H-iMidazole-2-carbonitrile synthesis
- Product Name:4-Methyl-1H-iMidazole-2-carbonitrile
- CAS Number:70631-95-7
- Molecular formula:C5H5N3
- Molecular Weight:107.11
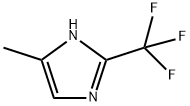
66675-23-8
5 suppliers
inquiry

70631-95-7
27 suppliers
inquiry
Yield:70631-95-7 61%
Reaction Conditions:
with ammonium hydroxide at 25 - 30; for 40 h;Inert atmosphere;
Steps:
1 Compound 14.2. 4-Methyl-lH-imidazole-2-carbonitrile
Compound 14.2. 4-Methyl-lH-imidazole-2-carbonitrile. Into a 250-mL round- bottom flask, which was purged and maintained with an inert atmosphere of nitrogen, was placed 4-methyl-2-(trifluoromethyl)-lH-imidazole (compound 14.1, 800 mg, 5.33 mmol) and 5% aqueous ammonium hydroxide (50 mL). The resulting solution was stirred for 40 h at 25- 30 °C, then the pH of the solution was adjusted to 5-6 with acetic acid. The aqueous phase was extracted with ethyl acetate (3 x 150 mL) and the combined organic extracts were washed with brine (2 x 50 mL) and aqueous Na2C03 (sat., 2 x 20 mL), dried (Na2SC>4), filtered, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with ethyl acetate:PE (1 :20-1 : 10) as the eluent to yield 350 mg (61 %) of the title compound as a white solid.
References:
WO2014/8197,2014,A1 Location in patent:Page/Page column 87; 88