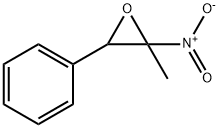
4-METHYL-2,5-DIPHENYLIMIDAZOLE synthesis
- Product Name:4-METHYL-2,5-DIPHENYLIMIDAZOLE
- CAS Number:2654-31-1
- Molecular formula:C16H14N2
- Molecular Weight:234.3
Yield:2654-31-1 88%
Reaction Conditions:
with indium(III) oxide;potassium carbonate in ethanol at 70; for 6 h;Green chemistry;Reagent/catalyst;Solvent;Temperature;
Steps:
Typical procedure for the synthesis of 5-Methyl-2,4-diphenyl-1H-imidazole
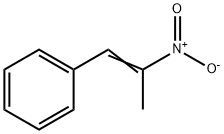
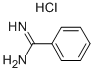
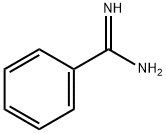
General procedure: In an oven dried 10 mL round bottom flask, benzamidinehydrochloride (78 mg, 0.5 mmol), (2-nitro-propenyl)-benzene (81 mg,0.5 mmol), nano In2O3 (7 mg, 5 mol %), K2CO3 (69 mg, 1 equiv), and dry EtOH(2 mL) were added and stirred for 6 h at 70 C. The progress of the reaction wasmonitored by TLC. After completion of the reaction the excess EtOH wasevaporated in a rotary evaporator and the crude reaction mixture was dilutedwith water (3 mL), and extracted with DCM. The organic layer was dried overanhydrous Na2SO4 and evaporated under vacuum. The residue was purified bycolumn chromatography (hexane/ethyl acetate as eluent) to afford theanalytically pure product as a white solid (103 mg, 88%). mp 180-184 C. IR(KBr) 3357, 3076, 2692, 2584, 1872, 1735, 1598, 1492, 1456, 1406, 1278, 1126,975 cm1; d 1H NMR (400 MHz, DMSO-d6): d 8.15 (d, J = 7.2 Hz, 2H), 7.72 (d,J = 7.2 Hz, 2H), 7.54-7.45 (m, 5H), 7.34 (t, J = 7.6 Hz, 1H), 2.47 (s, 3H); 13C NMR(DMSO-d6, 100 MHz): d 143.4, 132.1, 131.5, 129.9, 129.3, 129.0, 127.6, 127.3,127.0, 126.1, 11.5; Anal. Calcd for C16H4N2: C, 82.02; H, 6.02; N, 11.96%. Found:C, 82.17; H, 6.10; N, 12.02%.
References:
Mitra, Shubhanjan;Bagdi, Avik Kumar;Majee, Adinath;Hajra, Alakananda [Tetrahedron Letters,2013,vol. 54,# 36,p. 4982 - 4985] Location in patent:supporting information
92962-80-6
0 suppliers
inquiry

2654-31-1
4 suppliers
inquiry