
(4-METHYL-2-PHENYL-1,3-OXAZOL-5-YL)METHANOL synthesis
- Product Name:(4-METHYL-2-PHENYL-1,3-OXAZOL-5-YL)METHANOL
- CAS Number:248924-06-3
- Molecular formula:C11H11NO2
- Molecular Weight:189.21

22260-83-9
17 suppliers
$283.00/1g

248924-06-3
10 suppliers
inquiry
Yield:248924-06-3 92%
Reaction Conditions:
with lithium aluminium tetrahydride in diethyl ether at 0; for 1 h;Inert atmosphere;
Steps:
1-10
The 4-Methyl-2-phenyloxazole-5-Methyl formate (215mg,1.0mmol) dissolved in anhydrous ether, The reaction system was cooled to 0 ° C, then LiAlH4 (57mg,1.5mmol) was added in portions, at 0 ° C reaction after 1 hour added ice water inactivated,The mixture was extracted twice with ethyl acetate and after the organic layer was washed with water and saturated brine, it was dried over anhydrous sodium sulfate and Concentration under reduced pressure to obtain the crude product.The compound was purified by column chromatography to obtain 4-Methyl-2-phenyloxazole-5-methanol(174mg,92%). The intermediate was taken with similar manner for the preparation of the compound LCZ-003, benzylbromide was replaced with p- Bromobenzyl bromide (350mg,1.4mmol), at last it was purified by column chromatography to obtain a product of 5 -((4-bromobenzyloxy) methyl) -4-methyl-2-phenyloxazole (268mg,83%).
References:
CN103694195,2016,B Location in patent:Paragraph 0269; 0271
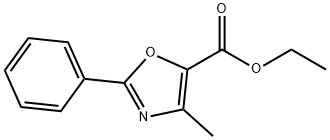
4620-52-4
10 suppliers
inquiry

248924-06-3
10 suppliers
inquiry
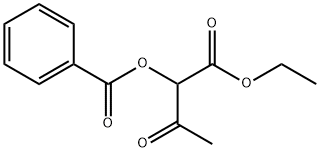
4620-46-6
6 suppliers
inquiry

248924-06-3
10 suppliers
inquiry
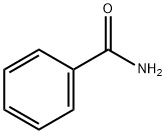
55-21-0
383 suppliers
$5.00/5G

248924-06-3
10 suppliers
inquiry
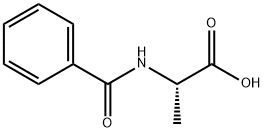
1205-02-3
121 suppliers
$14.00/1g

248924-06-3
10 suppliers
inquiry