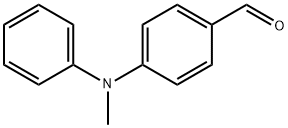
4-[METHYL(PHENYL)AMINO]BENZALDEHYDE synthesis
- Product Name:4-[METHYL(PHENYL)AMINO]BENZALDEHYDE
- CAS Number:55489-38-8
- Molecular formula:C14H13NO
- Molecular Weight:211.26
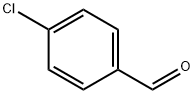
104-88-1
613 suppliers
$5.00/25g
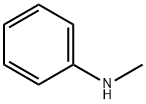
100-61-8
380 suppliers
$8.00/1g
![4-[METHYL(PHENYL)AMINO]BENZALDEHYDE](/CAS/GIF/55489-38-8.gif)
55489-38-8
19 suppliers
$45.00/10mg
Yield:55489-38-8 95%
Reaction Conditions:
Stage #1: 4-chlorobenzaldehyde;N-methylanilinewith sodium methylate;palladium diacetate;sodium sulfate;DavePhos for 1 h;Milling;Buchwald-Hartwig Coupling;
Stage #2: in water;ethyl acetate; for 0.0333333 h;Milling;Buchwald-Hartwig Coupling;
Steps:
2. General Procedure
General procedure: A mixture of substrate 2 (0.5 mmol), 1 (0.6 mmol), Pd(OAc)2 (2 mol%), Xphos (4 mol%), NaOtBu (2.0 equiv.) and Na2SO4 (2.0 g) were added to the 25 mL screw-capped stainless-steel vessel, along with two stainless steel balls ( = 1.4 cm). After that, the vessel was placed in the mixer mill, and the contents were ball milled at 30 Hz for 60 min. At the end of the reaction, small portion (3 mL) ethyl acetate and (3 mL) H2O were added in to the vessel and grinding for another 2 min at 30 Hz. Then, after the washing by brine, the organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo to give a residue, which was purified by flash column chromatography on silica gel to give the desired product.
References:
Shao, Qiao-Ling;Jiang, Zhi-Jiang;Su, Wei-Ke [Tetrahedron Letters,2018,vol. 59,# 23,p. 2277 - 2280] Location in patent:supporting information
88284-48-4
164 suppliers
$9.00/250mg
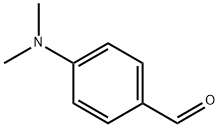
100-10-7
604 suppliers
$5.00/1g
![4-[METHYL(PHENYL)AMINO]BENZALDEHYDE](/CAS/GIF/55489-38-8.gif)
55489-38-8
19 suppliers
$45.00/10mg
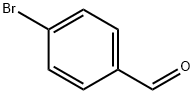
1122-91-4
672 suppliers
$9.00/10g

100-61-8
380 suppliers
$8.00/1g
![4-[METHYL(PHENYL)AMINO]BENZALDEHYDE](/CAS/GIF/55489-38-8.gif)
55489-38-8
19 suppliers
$45.00/10mg
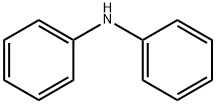
122-39-4
334 suppliers
$14.00/5g
![4-[METHYL(PHENYL)AMINO]BENZALDEHYDE](/CAS/GIF/55489-38-8.gif)
55489-38-8
19 suppliers
$45.00/10mg
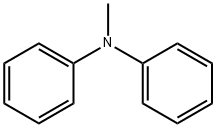
552-82-9
166 suppliers
$6.00/5g
![4-[METHYL(PHENYL)AMINO]BENZALDEHYDE](/CAS/GIF/55489-38-8.gif)
55489-38-8
19 suppliers
$45.00/10mg