
[4-(Methylamino)-3-nitrophenyl]methanol synthesis
- Product Name:[4-(Methylamino)-3-nitrophenyl]methanol
- CAS Number:62347-97-1
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18

1336-21-6
701 suppliers
$14.56/250ML
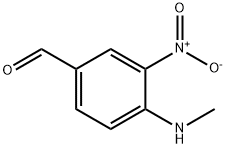
42564-41-0
21 suppliers
inquiry
![[4-(Methylamino)-3-nitrophenyl]methanol](/CAS/GIF/62347-97-1.gif)
62347-97-1
19 suppliers
inquiry
Yield:62347-97-1 1.7 g (56%)
Reaction Conditions:
with hydrogenchloride;sodium borohydrid
Steps:
2 Synthesis of Mono-2-flavo-β-cyclodextrin 12 STR14
4-Methylamino-3-nitro-benzylalcohol (9). 0.32 g (8.3 mmol) of sodium borohydride was added to 20 ml absolute alcohol solution containing 3.0 g (16.6 mmol) of 8. The suspension was stirred for 3 hours. The solution was then cooled in an ice/water bath, and 8.5 ml 2N HCl was added dropwise to the solution to decompose unreacted sodium borohydride. The pH of the solution was adjusted to 10 by adding about 10 ml of concentrated ammonium hydroxide and then extracted with 60 ml chloroform three times. After being dried over magnesium sulfate and evaporated, the residue was recrystallized from water to yield 1.7 g (56%) of orange 9. mp:126°-127° C. 1 H NMR: (DMSO-d6) δ 8.15 (s, 1H, NH), 8.01(s, 1H, H-2), 7.50 (d, 1H, J=8.6 Hz, H-5), 6.97p, (d, 1H, J=8.8 Hz, H-6), 5.22(s, 1H, OH), 4.41(s, 2H, CH2), 2.96(d, 3H, J=4.7 Hz, CH3). 13 C NMR:(DMSO-d6) δ 145.15, 135.70, 130.29, 129.30, 123.44, 114.20, 61.70, 29.69. Anal. Calcd for C8 H4 N2 O3:C, 52.74; H, 5.53; N, 15.38; O, 26.35; found: C, 53.02; H, 5.52; N, 15.48; O, 25.98.
References:
US5258370,1993,A

42564-41-0
21 suppliers
inquiry
![[4-(Methylamino)-3-nitrophenyl]methanol](/CAS/GIF/62347-97-1.gif)
62347-97-1
19 suppliers
inquiry
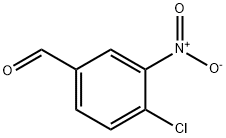
16588-34-4
175 suppliers
$6.00/1g
![[4-(Methylamino)-3-nitrophenyl]methanol](/CAS/GIF/62347-97-1.gif)
62347-97-1
19 suppliers
inquiry