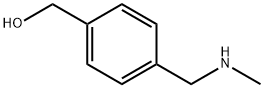
(4-Methylaminomethyl-phenyl)-methanol synthesis
- Product Name:(4-Methylaminomethyl-phenyl)-methanol
- CAS Number:132719-04-1
- Molecular formula:C9H13NO
- Molecular Weight:151.21
![4-[(tert-Butoxycarbonylamino)methyl]benzoic acid](/CAS/GIF/33233-67-9.gif)
33233-67-9
162 suppliers
$7.00/1g

132719-04-1
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-(tert-Butoxycarbonylaminomethyl)benzoic acidwith lithium aluminium tetrahydride in tetrahydrofuran at 20;Cooling with ice;Reflux;
Stage #2: with hydrogenchloride in water;
Steps:
2
Stage 2 - {4-[(methylamino)methyl|phenyl}methano.; To a cooled (ice bath) suspension of lithium aluminium hydride (5.03 g, 132 mmol) in tetrahydrofuran (100 mL) was added slowly a solution of the product of Stage 1 (5.34 g, 21 mmol) in tetrahydrofuran (20 mL). After addition was complete, the mixture was warmed to room temperature then heated at reflux for 96 hours. The mixture was then cooled to room temperature and excess lithium aluminium hydride quenched by careful addition of ethyl acetate (20 mL over 15 minutes) followed by slow addition of 2M HCl (100 mL). The mixture was extracted with ethyl acetate (2 times 100 mL).The aqueous layer was then basified by addition of sodium hydroxide and di-tert-butyl dicarbonate (9.0 g, 27 mmol) was added. The mixture was stirred at room temperature for 3 hours, then product was extracted with ethyl acetate (3 times 250 mL), the combined organic extracts were dried (MgS04) and concentrated. To the residue obtained (1.278 g, 16% yield) was added HCl (10 mL, 4M solution in dioxane). The solution was stirred at room temperature for 1 hour, then concentrated under vacuum. The solid obtained was washed with diethyl ether (2 times 50 mL), and dried to yield the desired product (0.890 g, 23% yield). LC/MS: m/z 152 [M+H]+
References:
WO2011/154708,2011,A1 Location in patent:Page/Page column 66
874-89-5
173 suppliers
$6.00/250mg

132719-04-1
2 suppliers
inquiry
39895-56-2
141 suppliers
$15.00/100mg

132719-04-1
2 suppliers
inquiry
![Acetamide, N-[[4-(hydroxymethyl)phenyl]methyl]-](/CAS/20210305/GIF/136869-07-3.gif)
136869-07-3
0 suppliers
inquiry

132719-04-1
2 suppliers
inquiry
56-91-7
513 suppliers
$5.00/1g

132719-04-1
2 suppliers
inquiry