
4-methylbenzo[d]oxazol-2(3H)-one synthesis
- Product Name:4-methylbenzo[d]oxazol-2(3H)-one
- CAS Number:78258-80-7
- Molecular formula:C8H7NO2
- Molecular Weight:149.15

2835-97-4
157 suppliers
$8.00/250mg
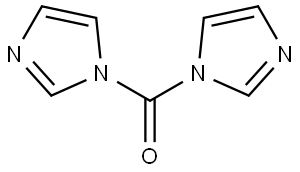
530-62-1
927 suppliers
$6.00/25g
![4-methylbenzo[d]oxazol-2(3H)-one](/CAS/GIF/78258-80-7.gif)
78258-80-7
19 suppliers
$70.68/50mgs:
Yield:78258-80-7 99%
Reaction Conditions:
in acetonitrile at 23 - 70;
Steps:
80
To a solution of 2-amino-3-methylphenol (777 mg, 6.32 mmol) in acetonitrile (27 mL) at 23° C. was added 1,1'-carbonyldiimidazole (3.07 g, 18.9 mmol). The reaction mixture was heated to 70° C., and stirred overnight. The reaction mixture was cooled to room temperature and partitioned between ethyl acetate (150 mL) and H2O (100 mL). The organic layer was separated and washed with brine (100 mL), dried (MgSO4), and concentrated to yield the crude product, which was purified by silica gel column chromatography (20-50% EtOAc in hexanes) to yield 4-methyl-3H-benzooxazol-2-one (931 mg, 99%).
References:
US2006/211603,2006,A1 Location in patent:Page/Page column 68
201230-82-2
1 suppliers
inquiry

4920-77-8
213 suppliers
$15.00/5g
![4-methylbenzo[d]oxazol-2(3H)-one](/CAS/GIF/78258-80-7.gif)
78258-80-7
19 suppliers
$70.68/50mgs:
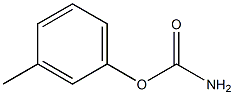
33222-69-4
0 suppliers
inquiry
![4-methylbenzo[d]oxazol-2(3H)-one](/CAS/GIF/78258-80-7.gif)
78258-80-7
19 suppliers
$70.68/50mgs:

22876-16-0
38 suppliers
$65.00/50mg
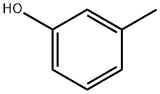
108-39-4
467 suppliers
$13.00/25g
![4-methylbenzo[d]oxazol-2(3H)-one](/CAS/GIF/78258-80-7.gif)
78258-80-7
19 suppliers
$70.68/50mgs:

22876-16-0
38 suppliers
$65.00/50mg
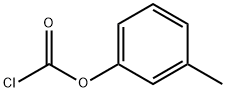
29430-39-5
17 suppliers
inquiry
![4-methylbenzo[d]oxazol-2(3H)-one](/CAS/GIF/78258-80-7.gif)
78258-80-7
19 suppliers
$70.68/50mgs:

22876-16-0
38 suppliers
$65.00/50mg