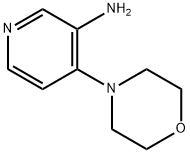
4-Morpholinopyridin-3-aMine synthesis
- Product Name:4-Morpholinopyridin-3-aMine
- CAS Number:90648-26-3
- Molecular formula:C9H13N3O
- Molecular Weight:179.22
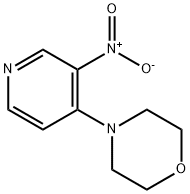
78070-09-4
0 suppliers
inquiry

90648-26-3
27 suppliers
$45.00/10mg
Yield:90648-26-3 84%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol; under 760.051 Torr; for 3 h;
Steps:
B Part B. 4-Morpholinopyridin-3 -amine
A mixture of 4-(3-nitropyridin-4-yl)mo holine (6.00 g, 28.7 mmol) and 10% palladium on carbon (1.526 g, 1.434 mmol) in MeOH (50 mL) was stirred under H2 at 1 atm for 3 h. The catalyst was removed by filtration through a pad of Celite. The mixture was concentrated and the residue was purified by column chromatography on silica gel (10% methanol in methylene chloride; 160 g column) to afford 4-morpholinopyridin-3-amine (4.30 g, 23.99 mmol, 84% yield) as an off-white solid: NMR (400MHz, DMSO-de) δ 7.94 (s, 1H), 7.76 (d, J=5.3 Hz, 1H), 6.78 (d, J=5.3 Hz, 1H), 4.84 (s, 2H), 3.82 - 3.70 (m, 4H), 2.94 - 2.85 (m, 4H); LCMS (ESI) mle 180.1 [(M+H) + , calcd for C9H14N3O 180.1].
References:
WO2018/98411,2018,A1 Location in patent:Page/Page column 91; 92

110-91-8
679 suppliers
$9.00/1g
20511-15-3
227 suppliers
$9.00/1g

90648-26-3
27 suppliers
$45.00/10mg
13091-23-1
339 suppliers
$14.14/1gm:

90648-26-3
27 suppliers
$45.00/10mg