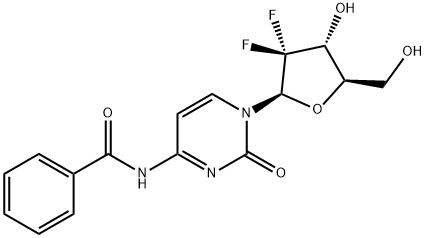
4-N-benzoylgeMcitabine synthesis
- Product Name:4-N-benzoylgeMcitabine
- CAS Number:142816-70-4
- Molecular formula:C16H15F2N3O5
- Molecular Weight:367.3

98-88-4
579 suppliers
$10.00/5g

122111-03-9
626 suppliers
$8.00/25mg

142816-70-4
12 suppliers
inquiry
Yield:142816-70-4 83%
Reaction Conditions:
Stage #1: gemcitabine hydrochloridewith ammonium sulfate;1,1,1,3,3,3-hexamethyl-disilazane for 2.5 h;Reflux;
Stage #2: benzoyl chloridewith 1-methyl-1H-imidazole in dichloromethane at 20; for 3 h;
Stage #3: with triethylamine in methanol;dichloromethane at 20; for 1.5 h;
Steps:
2 Synthesis of mPEGn-5'-Gemcitabine Monophosphate Conjugates
Example 2
Synthesis of mPEGn-5'-Gemcitabine Monophosphate Conjugates
N-Benzoyl-gemcitabine (1):
A mixture of gemcitabine hydrochloride (300 mg, 1 mmol), hexamethyldisilazane (5 ml), and a catalytic amount of ammonium sulfate (5 mg) in dioxane (5 ml) was heated under reflux for 2.5 hours.
The dioxane was evaporated and the reaction mixture was dissolved in toluene (20 ml) and then evaporated three times.
After removal of toluene, the residue was dissolved in dichloromethane (10 ml).
To this solution was added N-methylimidazole (0.24 ml, 3 mmol) and benzoyl chloride (0.35 ml, 3 mmol) and the solution was stirred for three hours at room temperature.
The reaction mixture was then concentrated to an oily residue, which was dissolved in a solution of triethylamine (3 ml) and methanol (20 ml).
This solution was stirred for 1.5 hours at room temperature.
The product was purified by flash chromatography (CH2Cl2/MeOH=2%?10%) to give N-benzoyl-gemcitabine 1 (303 mg, yield 83%).
1H NMR (MeOD): δ 8.25 (d, 1H), 7.81-7.80 (m, 2H), 7.56-7.22 (m, 4H), 6.14-6.04 (m, 1H), 4.21-4.10 (m, 1H), 3.83-3.80 (m, 2H), 3.72-3.61 (m, 2H).
References:
US9365604,2016,B2 Location in patent:Page/Page column 40; 41
93-97-0
374 suppliers
$6.00/25g

95058-81-4
526 suppliers
$6.00/100mg

142816-70-4
12 suppliers
inquiry

95058-81-4
526 suppliers
$6.00/100mg

142816-70-4
12 suppliers
inquiry