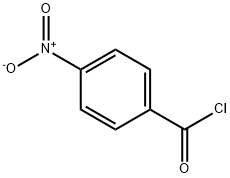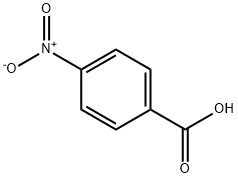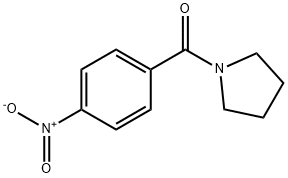
4-Nitro-1-(pyrrolidinocarbonyl)benzene synthesis
- Product Name:4-Nitro-1-(pyrrolidinocarbonyl)benzene
- CAS Number:53578-11-3
- Molecular formula:C11H12N2O3
- Molecular Weight:220.22
Yield:53578-11-3 159 mg
Reaction Conditions:
in acetonitrile at 20;Inert atmosphere;
Steps:
General procedure: To a solution of N-hydroxysuccinimide (115 mg, 1 mmol) in MeCN (1.5 mL), aldehyde (1 mmol) was added, and iodobenzene diacetate (322 mg, 1 mmol) was added in one portion, the reaction mixture was stirred for 2-3 min, after consumption of iodobenzene diacetate, amine (1 mmol) was then added under nitrogen atmosphere. The reaction was stirred for 1-15 hrs, until the consumption of NHSI ester was observed as judged by TLC. The crude reaction was quenched with water and extracted with EtOAc (5 mL ×3). The combined solvents were dried with MgSO4, filtered, and concentrated under vacuum, and the residue was purified by column chromatography (EtOAc/hexanes as eluent).
References:
Yao, Haoyi;Tang, Yun;Yamamoto, Kana [Tetrahedron Letters,2012,vol. 53,# 38,p. 5094 - 5098] Location in patent:supporting information; experimental part
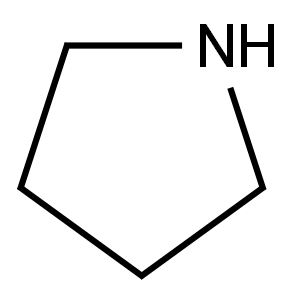
![2,5-Pyrrolidinedione, 1-[(4-nitrobenzoyl)oxy]-](/CAS/20180601/GIF/30364-58-0.gif)