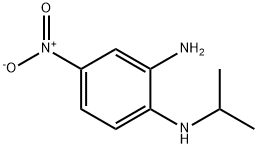
4-nitro-N1-(propan-2-yl)benzene-1,2-diamine synthesis
- Product Name:4-nitro-N1-(propan-2-yl)benzene-1,2-diamine
- CAS Number:56136-70-0
- Molecular formula:C9H13N3O2
- Molecular Weight:195.22
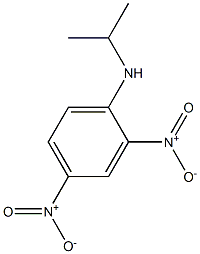
13059-85-3
7 suppliers
inquiry

56136-70-0
4 suppliers
inquiry
Yield:56136-70-0 708 mg
Reaction Conditions:
with formic acid;palladium 10% on activated carbon;triethylamine in acetonitrile at -15 - 60; for 7 h;Inert atmosphere;
Steps:
48A N1-isopropyl-4-nitrobenzene-1,2-diamine
General procedure: 1.20 g (5.68 mmol) of N-ethyl-2,4-dinitroaniline from Example 42A were initially charged in 3 ml of acetonitrile under argon and 64 mg (0.06 mmol) of palladium (10% on activated carbon) and 3.40 ml (24.38 mmol) of triethylamine were added. The reaction mixture was cooled to -15° C., and a solution of 1.03 ml (27.44 mmol) of formic acid in 3 ml of acetonitrile was added. The reaction mixture was stirred at 40° C. for 1 h and at 60° C. for 2 h. For workup, the reaction mixture at RT was filtered through kieselguhr and washed with ethyl acetate/methanol (1:1), and the filtrate was concentrated. The residue was admixed with water, and the precipitated solid was filtered off with suction, washed with water and dried at 50° C. under reduced pressure. This gave 546 mg (47% of theory) of the title compound. The preparation and purification of the target compound were analogous to Example 43A, with a reaction time of 7 h. Proceeding from 1.13 g (5.01 mmol) of N-isopropyl-2,4-dinitroaniline from Example 47A, 708 mg (72% of theory) of the title compound were obtained. LC-MS (Method 1): Rt=0.88 min; MS (ESIpos): m/z=196 (M+H)+. 1H NMR (400 MHz, DMSO-d6): δ [ppm]=1.21 (d, 6H), 3.69-3.81 (m, 1H), 5.11-5.24 (m, 2H), 5.62 (d, 1H), 6.49 (d, 1H), 7.39 (d, 1H), 7.51 (dd, 1H).
References:
US2015/148340,2015,A1 Location in patent:Paragraph 0687; 0706; 0707; 0708; 0709
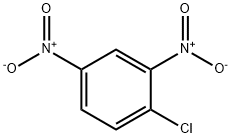
97-00-7
12 suppliers
$17.67/10gm:

56136-70-0
4 suppliers
inquiry