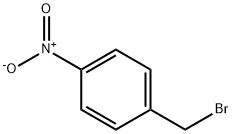
4-NITROBENZYL BROMOACETATE synthesis
- Product Name:4-NITROBENZYL BROMOACETATE
- CAS Number:16869-24-2
- Molecular formula:C9H8BrNO4
- Molecular Weight:274.07

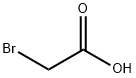
619-73-8
576 suppliers
$5.00/10g
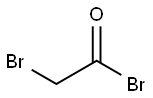
598-21-0
312 suppliers
$10.00/10g

16869-24-2
43 suppliers
$29.10/2g
Yield:16869-24-2 86%
Reaction Conditions:
with collidine in dichloromethane at 0 - 20; for 24 h;
Steps:
5.g The synthesis of [Step g] 4-nitrobenzyl 2-bromoacetate(Compound 26)
4nitrobenzylalcohol (3.06 g, 20 mmol) andcollidine (2.9 mL, 22 mmol) was dissolved in dichloromethane (100mL), 0 ° C under was added bromoacetyl bromide (1.81mL, 20 mmol), and reacted for 24 hours at room temperature. The solvent was evaporated under reduced pressure, diethylether was added to the residue was removed by filtration of the precipitated crystals. After concentrating the filtrate, theresidue was purified by silica gel column chromatography (hexane / ethyl acetate) to give compound 26 (4.73 g, 86% yield).
References:
JP2015/63503,2015,A Location in patent:Paragraph 0100; 0107