
4-(Nitrooxy)butan-1-ol synthesis
- Product Name:4-(Nitrooxy)butan-1-ol
- CAS Number:22911-39-3
- Molecular formula:C4H9NO4
- Molecular Weight:135.12
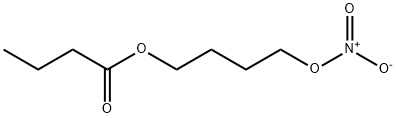
1100273-14-0
4 suppliers
inquiry

22911-39-3
14 suppliers
inquiry
Yield: 72.5%
Reaction Conditions:
with methanol;sodium hydroxide;water at 20; for 1.75 h;
Steps:
3; 5
Example 3; Preparation of 4-Nitrooxybutan-l-ol (compound Ia); 4-Nitrooxybutan-l-ol butyrate (1350 g, 95 % w/w, 6.25 mol) was added at room temperature to a mixture of methanol (1930 ml) and water (515 ml). Sodium hydroxide (30 %, 911 g, 6.83 mol)0 was added with stirring over 45 min and the resulting reaction mixture was allowed to stir at room temperature for 1 hour. Sulphuric acid (5 %, 300 ml) was added which gave a pH of 7 - 8. Methanol was removed completely by vacuum distillation (80 -
References:
NICOX S.A. WO2009/723, 2008, A1 Location in patent:Page/Page column 23-24; 25
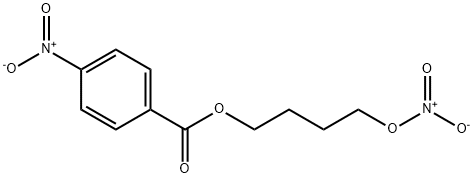
1141845-41-1
0 suppliers
inquiry

22911-39-3
14 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

22911-39-3
14 suppliers
inquiry
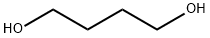
110-63-4
685 suppliers
$24.80/250g

22911-39-3
14 suppliers
inquiry
