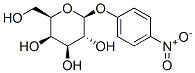
4-Nitrophenyl-beta-D-galactopyranoside synthesis
- Product Name:4-Nitrophenyl-beta-D-galactopyranoside
- CAS Number:3150-24-1
- Molecular formula:C12H15NO8
- Molecular Weight:301.25
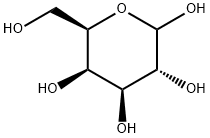
10257-28-0
74 suppliers
$138.00/100g
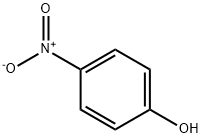
100-02-7
5 suppliers
$11.00/5G

108-24-7
5 suppliers
$14.00/250ML
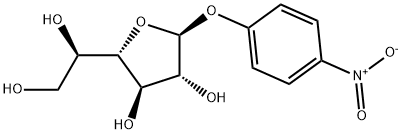
100645-45-2
31 suppliers
inquiry

3150-24-1
265 suppliers
$22.39/1G
Yield:-
Reaction Conditions:
Stage #1: D-Galactosewith pyridine at 70; for 1 h;
Stage #2: acetic anhydride at 23 - 70; for 25 h;
Stage #3: 4-nitro-phenolOverall yield = 1.32 g; Further stages;
Steps:
3.2 Isomerization and acetylation to obtain per-acetylated galactofuranose (4)
General procedure: To 9 pyridine (30mL) was added d-galactose (1.81g, 10.0mmol) while stirring at 70°C. After 1h, acetic anhydride (6.00mL, 63.5mmol) was added into a reaction mixture at 70°C and the mixture was stirred for 1h. Then the reaction mixture was cooled to 23°C, stirred for 24h, and then concentrated in vacuo. The resulting syrup was diluted with EtOAc (100mL), washed with water (100mL), 1m aq. HCl (100mL), and brine, followed by drying over MgSO4. Concentration in vacuo gave a mixture of per-acetylated galactofuranose (4) and per-acetylated galactopyranose (4p) in 96% yield (3.75g, 9.60mmol). 1H NMR (CDCl3): δ5.73-5.70 (m, 0.6H, H-14p), 5.43-5.41 (m, 0.4H, H-14), 5.35-5.28 (m, 1H, H-3), 5.08-5.02 (m, 1H, H-2), 4.37-4.31 (m, 1H, H-4), 4.18-4.16 (m, 1H, H-5), 4.13-4.08 (m, 2H, H-6), 2.16 (s, 3H, Ac), 2.12 (s, 3H, Ac), 2.10 (s, 3H, Ac), 2.04 (s, 3H, Ac), 1.99 (s, 3H, Ac); 13C NMR (CDCl3): δ 170.3, 170.1, 169.9, 169.8, 169.4, 169.0, 99.1, 93.0, 92.1, 89.7, 82.1, 80.6, 79.0, 76.3, 75.3, 73.3, 71.7, 70.8, 70.3, 69.2, 68.7, 67.8, 67.4, 67.3, 66.8, 66.4, 62.5, 62.1, 61.2, 61.0, 21.0, 20.9, 20.8, 20.7, 20.6, 20.5, 20.4; Anal. Calc. for: C16H22O11: C, 49.23; H, 5.68. Found: C, 49.28; H, 5.87 To a solution of 4 and 4p (2:3, 3.72g, 9.55mmol) with MS4A in CH3CN (50mL) was added p-nitrophenol (3.99g, 28.7mmol) and BF3·Et2O (3.63mL, 28.67mmol) at 0°C. The reaction mixture was stirred at the same temperature for 1h and then at 23°C for 24h. Next the mixture was filtered through a Celite pad and the filtrate was concentrated in vacuo. The residue was diluted with EtOAc (100mL), washed with sat. aq. NaHCO3 solution (100mL x 8) to remove excess p-nitrophenol as a sodium salt and brine (50mL), dried over MgSO4, and concentrated in vacuo to dryness, producing a mixture of 42 p-nitrophenyl 2,3,4,6-tetra-O-acetyl-β-d-galactofuranoside (5) and 13 p-nitrophenyl 2,3,4,6-tetra-O-acetyl-β-d-galactopyranoside (5p) as a brown syrup (3.77g, 8.02mmol, 84%). The mixture of 5 and 5p was used for deprotection and hydrolysis without any purification. 1H NMR (CDCl3): δ 8.21 (m, 2H, Jo,m=9.2Hz, Ph(m)), 7.19 (d, 2H, Jo,m=9.2Hz, Ph(o)), 5.88 (d, 1H, J1,2=4.0Hz, H-1), 5.57-5.54 (m, 1H, H-4), 5.53-5.52 (m, 1H, H-5), 5.31 (dd, 1H, J2,3=4.0Hz, J3,4=10.8Hz, H-3), 4.25 (t, 1H, J1,2=J2,3=4.0Hz, H-2), 4.13-4.03 (m, 2H, H-6), 2.18 (s, 3H, Ac), 2.08 (s, 3H, Ac), 2.04 (s, 3H, Ac), 1.93 (s, 3H, Ac); 13C NMR (CDCl3): δ 170.4, 170.3, 170.2, 170.1, 170.0, 169.9, 169.6, 169.3, 160.7, 142.8, 126.2, 125.8, 116.5, 103.7, 98.6, 94.7, 89.7, 81.8, 81.2, 76.2, 69.1, 68.7, 67.8, 67.2, 66.4, 62.4, 61.3, 61.2, 20.9, 20.8, 20.7, 20.6, 20.5; Anal. Calc. for: C20H23NO12+H2O: C, 47.81; H, 4.81; N, 2.79. Found: C, 47.59; H, 4.89; N, 2.67. To a solution of 5 and 5p (2.07 g, 4.41 mmol) in CH3OH (10 mL)was added 28% aq. NH3 solution (10 mL) at 0 °C. The resulting solutionwas stirred at the same temperature for 1 h and then at 23 °C for 24 h.The reaction solution was concentrated using a rotary evaporator(35 °C), and the resulting syrup was crystalized with Et2O to afford themixture of p-nitrophenyl β-D-galactofuranoside (1) and p-nitrophenyl β-D-galactopyranoside (1p) as a brown solid (1.32 g, 4.39 mmol, 99%).To a solution of the above mixture of 1 and 1p (0.58 g, 1.9 mmol) in50mM phosphate buffer (pH 7.2, 20 mL) was added β-galactosidase fromEscherichia coli (FUJIFILM Wako Pure Chemical Corporation,>600Units/mg, 1.0 mg) followed by stirring at 37 °C for 24 h. After confirmingselective pyranoside hydrolysis with TLC analysis, the reaction mixturewas washed with CHCl3 (20 mL x 3), and then concentrated in vacuo.Target material was purified by silica-gel column chromatography(CHCl3:CH3OH, 4/1) to give p-nitrophenyl β-D-galactofuranoside (pNPGalf,1) as a colorless solid (0.25 g, 0.83 mmol, 43%).
References:
Ota, Ryo;Okamoto, Yumi;Vavricka, Christopher J.;Oka, Takuji;Matsunaga, Emiko;Takegawa, Kaoru;Kiyota, Hiromasa;Izumi, Minoru [Carbohydrate Research,2019,vol. 473,p. 99 - 103]

10257-28-0
74 suppliers
$138.00/100g

100-02-7
5 suppliers
$11.00/5G

3150-24-1
265 suppliers
$22.39/1G
4163-60-4
345 suppliers
$6.00/5g

3150-24-1
265 suppliers
$22.39/1G
17042-39-6
38 suppliers
inquiry

3150-24-1
265 suppliers
$22.39/1G

100-02-7
5 suppliers
$11.00/5G

3150-24-1
265 suppliers
$22.39/1G