
4-(OXETAN-3-YL)BENZONITRILE synthesis
- Product Name:4-(OXETAN-3-YL)BENZONITRILE
- CAS Number:1044507-48-3
- Molecular formula:C10H9NO
- Molecular Weight:159.18
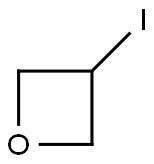
26272-85-5
231 suppliers
$10.00/1g
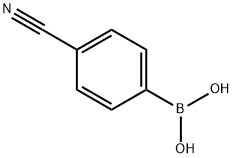
126747-14-6
395 suppliers
$6.00/1g

1044507-48-3
7 suppliers
inquiry
Yield:1044507-48-3 79%
Reaction Conditions:
Stage #1: 4-cyanophenylboronic acidwith nickel(II) iodide;sodium hexamethyldisilazane;trans-2-aminocyclohexanol hydrochloride in isopropyl alcohol; for 0.166667 h;Inert atmosphere;
Stage #2: 3-iodooxetane in isopropyl alcohol at 80; for 0.5 h;Microwave irradiation;Inert atmosphere;
Steps:
1 4-(Oxetan-3-yl)benzonitrile (X12)
4-(Oxetan-3-yl)benzonitrile (X12)
A mixture of 4-cyanophenylboronic acid, X11 (294 mg, 2.0 mmol, 2.0 eq.), nickel (II) iodide (31.5 mg, 0.10 mmol, 0.10 eq.), sodium hexamethyldisilazane (366.7 mg, 2.0 mmol, 2.0 eq.) and trans-2-aminocyclohexanol hydrochloride (15.2 mg, 0.10 mmol, 0.10 eq.) in isopropanol (4.0 mL) was stirred under nitrogen.
After 10 min, a solution of 3-iodooxetane (184 mg, 1.0 mmol, 1.0 eq.) in isopropanol (0.25 mL) was added.
The resulting mixture was subjected to a microwave reactor at 80° C.
After 30 min, the mixture was diluted with EtOH and filtered through a Celite pad which was washed thoroughly with EtOH.
The filtrate was concentrated under reduced pressure.
The crude material was purified by flash column chromatography on silica gel (0-60% EtOAc/hexanes) to provide the title compound as a crystalline solid (120 mg, 79% yield). ES-MS [M+1]+: 160.2; 1H NMR (400 MHz, DMSO-d6) 7.77 (ddd, J=8.4, 1.8, 1.8 Hz, 2H), 7.50 (ddd, J=8.2 Hz, 1.8, 1.8 Hz, 2H), 4.88 (dd, J=8.3, 6.0 Hz, 2H), 4.53 (dd, J=6.4, 6.4 Hz, 1H), 4.27 (m, 1H).
References:
US2017/369505,2017,A1 Location in patent:Paragraph 0721; 0722

26272-85-5
231 suppliers
$10.00/1g
623-00-7
469 suppliers
$6.00/10g

1044507-48-3
7 suppliers
inquiry