
4-OXOCYCLOHEXANECARBONYL CHLORIDE synthesis
- Product Name:4-OXOCYCLOHEXANECARBONYL CHLORIDE
- CAS Number:914637-80-2
- Molecular formula:C7H9ClO2
- Molecular Weight:160.6
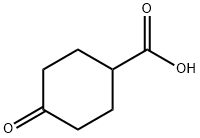
874-61-3
171 suppliers
$7.00/250mg

914637-80-2
9 suppliers
$279.23/1gm:
Yield:-
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20;
Steps:
C-01.a1
Oxalyl chloride (0.89 g, 7.0 mmol) and a few drops of dry DMF are added to a stirred solution of 4-oxo-cyclohexanecarboxylic acid (0.50 g, 3.5 mmol) in CH2Cl2 (7 ml) and the reaction is kept stirring at rt overnight. The volatiles are removed under reduced pressure, the residue is azeotroped with toluene and dried under high vacuum yielding crude acid chloride. This material is reacted without further purification with N-methylaniline (0.16 g, 1.5 mmol) in CH2Cl2 (7 ml) in the presence of DIEA (0.58 g, 4.5 mmol). After stirring overnight at rt, saturated aqueous NaHCO3 is added and the mixture extracted three times with CH2Cl2. The combined organic layers are dried over Na2SO4 and evaporated. The residue is column chromatographed on silica gel (hexane/ EtOAc 2:1) and the title EPO
References:
WO2006/70325,2006,A2 Location in patent:Page/Page column 72-73

6297-22-9
149 suppliers
$7.00/1g

914637-80-2
9 suppliers
$279.23/1gm:

17159-79-4
335 suppliers
$5.00/1g

914637-80-2
9 suppliers
$279.23/1gm: