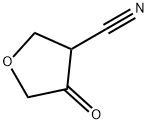
4-Oxotetrahydrofuran-3-carbonitrile synthesis
- Product Name:4-Oxotetrahydrofuran-3-carbonitrile
- CAS Number:856945-68-1
- Molecular formula:C5H5NO2
- Molecular Weight:111.1
Yield: 45.2%
Reaction Conditions:
Stage #1:glycolic acid methyl ester with potassium tert-butylate in tetrahydrofuran at 0; for 0.166667 h;
Stage #2:acrylonitrile in tetrahydrofuran at 20; for 3 h;
Steps:
P108.A
Preparation of 4-oxotetrahydrofuran-3-carbonitrile: To a suspension ofKOtBu (996.6 mg, 8.881 mmol) in THF (640.4 mg, 8.881 mmol) cooled to 0 °C was added dropwise methyl 2-hydroxyacetate (675.7 μ,, 8.881 mmol) and stirred for 10 minutes. The acrylonitrile (589.1 μ, 8.881 mmol) was then added and the reaction stirred at ambient temperature. After 3 hours, the reaction was diluted with H20 (50 mL), then extracted with Et20 (25 mL) to remove any starting ester. The basic aqueous phase was acidified with 2M HCl (5 mL), then extracted with Et20 (2 x 50 mL). The combined organic phases were dried with MgS04, filtered, and concentrated to afford a light brown oil (446 mg, 45.2% yield). 1H NMR (CDC13) δ 4.63 (t, 1H), 4.24 (t, 1H), 4.14 (d, 1H), 4.02 (d, 1H), 3.57 (t, 1H).
References:
ARRAY BIOPHARMA INC.;ALLEN, Shelley;ANDREWS, Steven, W.;BLAKE, James, F.;CONDROSKI, Kevin, R.;HAAS, Julia;HUANG, Lily;JIANG, Yutong;KERCHER, Timothy;KOLAKOWSKI, Gabrielle R.;SEO, Jeongbeob WO2012/158413, 2012, A2 Location in patent:Page/Page column 101

