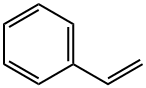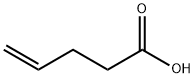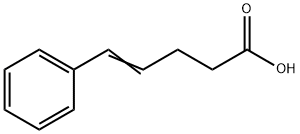
4-Pentenoic acid, 5-phenyl- synthesis
- Product Name:4-Pentenoic acid, 5-phenyl-
- CAS Number:28525-69-1
- Molecular formula:C11H12O2
- Molecular Weight:176.21
Yield:28525-69-1 95%
Reaction Conditions:
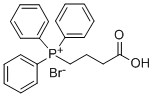
Stage #1: (3-carboxypropyl)(triphenyl)phosphonium bromidewith sodium hexamethyldisilazane in tetrahydrofuran at 0; for 0.5 h;
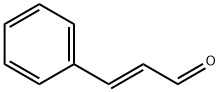
Stage #2: benzaldehyde in tetrahydrofuran at -78 - 20;
Steps:
(4E)-5-phenylpent-4-enoic acid, 19a
To a solution of (3-carboxypropyl)triphenylphosphonium bromide (1.9 g, 4.5 mmol, 1.1 eq.) in tetrahydrofuran (20 ml) was added dropwise a solution of sodium bis(trimethylsilyl)amide (1.0 M in tetrahydrofuran, 9.0 ml, 9.0 mmol, 2.2 eq.) at 0 cC. The solution was stirred for 30 min, then cooled to -78 °C. Benzaldehyde (0.42 ml, 4.2 mmol, 1 eq.) was then added dropwise. The reaction was allowed to warm to room temperature overnight. Water and ether were added. The water layer was separated and acidied with 1 M aqueous hydrochloric acid to pH = 1, then extracted twice with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered and concentrated in vacuo. The crude product was chromatographed on silica gel (3:1 ether:hexanes) to give 0.70 g of product (95%) as a white solid.
References:
Perkins, Robert J.;Xu, Hai-Chao;Campbell, John M.;Moeller, Kevin D. [Beilstein Journal of Organic Chemistry,2013,vol. 9,p. 1630 - 1636] Location in patent:supporting information