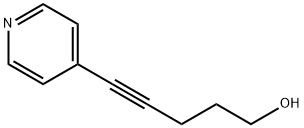
4-Pentyn-1-ol, 5-(4-pyridinyl)- synthesis
- Product Name:4-Pentyn-1-ol, 5-(4-pyridinyl)-
- CAS Number:191725-48-1
- Molecular formula:C10H11NO
- Molecular Weight:161.2
Yield:191725-48-1 82%
Reaction Conditions:
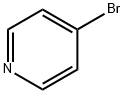
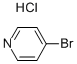
Stage #1: 4-bromopyridine hydrochloridewith sodium hydroxide in ethyl acetate;
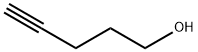
Stage #2: pent-1-yn-5-olwith bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide;triethylamine for 0.333333 h;Reflux;Sonogashira Cross-Coupling;
Steps:
Synthesis of 10f and 10g
General procedure: 4-Bromopyridine hydrochloride (2.5 g) was partitioned between 1 N sodium hydroxide (20 mL) and ethyl acetate (3×20 mL). The organic layer was separated, dried over Na2SO4 and concentrated under vacuum. The resulting oil was dissolved in Et3N (2.6 M) and degassed under nitrogen. 4-Pentyn-1-ol or 5-hexyn-1-ol (1.1 equiv) was added followed by bis(triphenylphosphine)palladium(II) chloride (0.01 equiv) and copper(I) iodide (0.02 equiv) and the reaction mixture stirred at reflux for 20 min. Aqueous work-up (ethyl acetate/H2O) and purification by chromatography on silica gel (gradient 0-30% ethyl acetate in heptane) led to 9f and 9g in 82% and 21% yield, respectively. 9f: 1H NMR (400MHz, CDCl3) δ 8.52 (m, 2H), 7.25 (m, 2H), 3.82 (m, 2H), 2.58 (t, J=7.2Hz, 2H), 1.88 (m, 2H), 1.62 (m, 1H). 9g: 1H NMR (400MHz, CDCl3) δ 8.52 (m, 2H), 7.25 (m, 2H), 3.73 (m, 2H), 2.48 (t, J=7.2Hz, 2H), 1.74 (m, 4H), 1.51 (m, 1H). Compound 9f or 9g was dissolved in acetic acid (0.05 M) and the solution hydrogenated using a H-Cube continuous-flow hydrogenation reactor (ThalesNano) (20% Pd(OH)2/C cartridge, 100 bars H2, 90°C, 1mL/min). Once the hydrogenation was complete, the reaction mixture was concentrated and dried under vacuum. The resulting crude was dissolved in CH2Cl2 (0.4 M) and reacted with Et3N (1.5 equiv) and di-t-butyl dicarbonate (1.2 equiv) at rt for 30 min. After aqueous work-up (CH2Cl2/H2O) and purification by chromatography on silica gel (gradient 0-30% ethyl acetate in heptane), compounds 10f and 10g were isolated in 80% yield. 10f: 1H NMR (400MHz, CDCl3) δ 4.06 (s, 2H), 3.64 (t, J=6.8Hz, 2H), 2.66 (t, J=11.4Hz, 2H), 1.54-1.66 (m, 4H), 1.45 (s, 9H), 1.24-1.39 (m, 8H), 1.08 (m, 2H). 10g: 1H NMR (400MHz, CDCl3) δ 4.05 (s, 2H), 3.64 (dd, J=6.2, 10.6Hz, 2H), 2.63 (t, J=12.0Hz, 2H), 1.53-1.65 (m, 4H), 1.45 (s, 9H), 1.20-1.40 (m, 10H), 1.04 (m, 2H).
References:
Bazin, Hélène G.;Li, Yufeng;Khalaf, Juhienah K.;Mwakwari, Sandra;Livesay, Mark T.;Evans, Jay T.;Johnson, David A. [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 6,p. 1318 - 1323]

14221-01-3
648 suppliers
$22.00/1g

10097-32-2
2 suppliers
inquiry
19524-06-2
483 suppliers
$6.00/5g
181878-28-4
0 suppliers
inquiry

191725-48-1
0 suppliers
inquiry