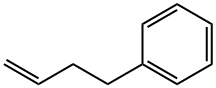
4-Phenyl-1-butene synthesis
- Product Name:4-Phenyl-1-butene
- CAS Number:768-56-9
- Molecular formula:C10H12
- Molecular Weight:132.2
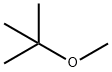
1634-04-4
655 suppliers
$14.00/100g

100-44-7
630 suppliers
$13.50/250G

107-05-1
400 suppliers
$16.80/100ML

768-56-9
123 suppliers
$53.00/5mL
Yield:768-56-9 93%
Reaction Conditions:
with sulfuric acid;nitrogen in tetrahydrofuran;water;iodine
Steps:
2 Example 2
Example 2 Into a 3 L four-necked flask equipped with a stirrer, a thermometer, a condenser and a dropping funnel, 37.45 g (1.54 mole) of metal magnesium in a shaved form and 0.1 g of iodine were charged. After the flask was filled with nitrogen, 500 ml of tetrahydrofuran and 500 ml of tert.-butyl methyl ether were added. The flask was sufficiently cooled on an ice bath, and then 118 g (1.54 mole) of allyl chloride was added dropwise at a temperature of 0° to 20° C. over a period of 2 hours with stirring. The reaction mixture obtained was filtrated at a room temperature under a nitrogen atmosphere to remove unreacted magnesium. The flitrate was transferred to another 3 L four-necked flask equipped with a stirrer, a thermometer, a condenser and a dropping funnel. Then 130 g (1.03 mole) of benzyl chloride was added dropwise at the same temperature over a period of 30 minutes, followed by stirring the mixture at the same temperature for 4 hours. After the completion of the reaction, the reaction mixture was added to 300 ml of 5% sulfuric acid at a temperature of 0° to 10° C., followed by stirring for 30 minutes. Thereafter it was allowed to stand and phase-separated. The resulting oil layer was washed with 200 ml of water, followed by phase-separation. The resulting oil layer was distilled in the similar manner to that in Example 1 to obtain 125.7 g of 4-phenyl-1-butene (yield 93%). An analysis of the product by gas chromatography indicated the purity of 98.0%.
References:
Sumitomo Chemical Company, Limited US5637736, 1997, A
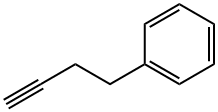
16520-62-0
82 suppliers
$13.00/250mg

768-56-9
123 suppliers
$53.00/5mL
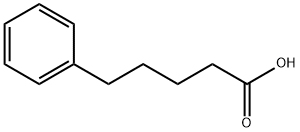
2270-20-4
173 suppliers
$11.00/1g

768-56-9
123 suppliers
$53.00/5mL

100-39-0
425 suppliers
$10.00/10 g

106-95-6
418 suppliers
$10.00/5g

768-56-9
123 suppliers
$53.00/5mL

100-44-7
630 suppliers
$13.50/250G

107-05-1
400 suppliers
$16.80/100ML

768-56-9
123 suppliers
$53.00/5mL